What are Neurofilaments?
Neurofilaments are cytoskeletal proteins that are highly specific for neurons. They comprise 85% of neuronal structural proteins and determine axonal diameter. Neurofilaments are present in both the central nervous system and peripheral nervous system neurons. There are several different types of neurofilaments, including heavy chain, medium chain, and light chain.
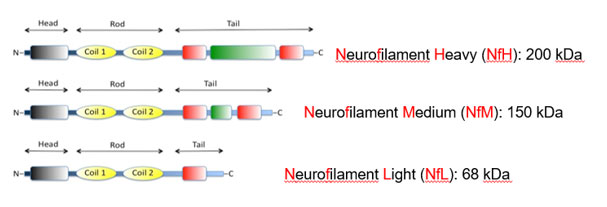
The half-life of neurofilaments in neurons is about 2-3 months, indicating a slow (but non-zero) turn-over inside neurons. Thus, even in healthy adults, neurofilaments are measurable in brain and body fluids. Neurofilament light (NfL) is the most studied neurofilament in human disease and the most commonly-reported neurofilament in research publications. Since neurofilaments are released from neurons due to almost any type of injury or disease, virtually all disorders and diseases of the CNS or PNS which disrupt neurons can lead to release of neurofilaments into the CSF (for the CNS) or blood (for the PNS). Whether neurofilaments are released by direct passive consequence of neuronal membrane disruption or through the known pathway for active secretion of other neuronal proteins is unknown. Once neurofilaments are released into the CSF, they will eventually flow into the periphery and are measurable in both plasma and serum. See Yuan and Nixon, Frontiers in Neuroscience 2021 for more background about neurofilaments.
How is NfL measured?
NfL can be measures from essentially any body fluid and is typically measured from the CSF, serum, or plasma. Early NfL assays utilized either enzyme-linked immunosorbent assay (ELISA) or electrochemiluninescence (ECL) technology. These technologies were unable to measure the low levels of NfL present in the blood of many individuals (called a “floor” effect) and thus had limited utility in assessing NfL outside of CSF. Single molecule array (SIMOA) technology is a digital methodology that provides very high assay sensitivity and thus essentially can measure almost any level of NfL found in plasma and serum. Several companies provide sensitive assays for NfL, including Quanterix, Siemens, and Roche Diagnostics/Labcorp. Each use a somewhat different approach and thus yields somewhat different values, making direct comparisons across assays difficult. Other assays are in development, too.
Do levels of NfL in serum relate to levels of NfL in CSF?
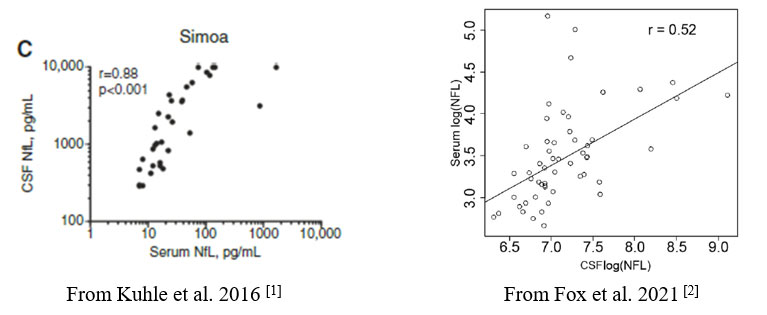
Most of NfL in serum and plasma are thought to derive from NfL in the CSF. Thus, it is not surprising that NfL in CSF correlates with serum, although that correlation differs between studies. One study including a broad range of MS patients found a correlation of r=0.88 [1] while another study involving only progressive MS patients found a correlation of only r=0.52 [2].
Figure 1. CSF/Serum Correlations in MS patients
How is NfL related to MS?
In MS, focal inflammation causes CNS injury and subsequent release of NfL. Thus, elevated levels of NfL are seen in the serum of patients who have gadolinium-enhancing lesions on MRI and clinical relapses. In a longitudinal study of monthly MRI and blood draws after stopping an MS therapy (natalizumab), increases in serum NfL levels were observed one month or more after development of enhancing lesions [3]. Additionally, not all patients with enhancing lesions in that study demonstrated elevations in NfL. An increase in NfL was not seen in patients without gadolinium-enhancing lesions, although would be expected in other conditions that injure CNS tissue such as stroke and trauma. Thus, NfL is a relatively specific indicator of active, focal inflammation in MS patients, but is not a very sensitive measure of that inflammation.
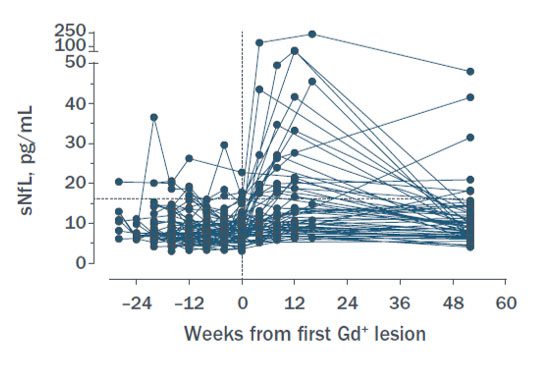
Figure 2. Serum NfL levels in MS patients who developed gadolinium-enhancing lesions, indexed from the time of first development of the first enhancing lesion (n=65). [3]
What level of NfL is considered abnormal?
Levels of NfL in otherwise healthy adults increases with age, renal insufficiency, and medical conditions such as diabetes. [4] Furthermore, different NfL assays have defined abnormal cut-offs quite differently. Not surprisingly, the expected value of NfL in MS patients without active inflammation is not well defined. Thus, identifying a specific abnormal cut-off in MS patients is challenging and current a topic of research. Online nomograms to define normal cut-offs have been proposed, but are not yet in routine use as this field is evolving.[4]
Does NfL predict disease activity in MS?
At a group level, elevations in serum NfL are associated subsequent clinical relapses and new gadolinium-enhancing and T2 lesions on MRI.[[5]; [6]; [7]] Whether levels of NfL are a better predictor of future disease activity than other known factors such as recent clinical relapses and new/active lesions on MRI is less clear.
Does NfL predict disease progression in MS?
Many studies have reported an association between elevated serum NfL and subsequent disability progression, conversion to secondary progressive MS, progressive brain atrophy, and worsening of cognitive performance.[[5] [7]; [8]; [9]] These studies provided encouragement that NfL may reflect the insidious neuroaxonal loss that is the presumptive underpinning of progressive MS. However, subsequent studies found that when focal inflammation is removed from the picture by studying patients receiving a highly-effective anti-inflammatory therapy such as natalizumab, serum NfL levels no longer correlate or predict subsequent disability progression.[[10]; [11]] Thus, the association reported in previous studies appear likely to have been driven by the focal inflammation represented by new MRI lesions and clinical relapses. Accordingly, NfL appears to reflect the focal inflammation (e.g. clinical relapses and new/active MRI lesions) sometimes seen in progression MS and does not appear to reflect the ongoing pathology of progressive MS.
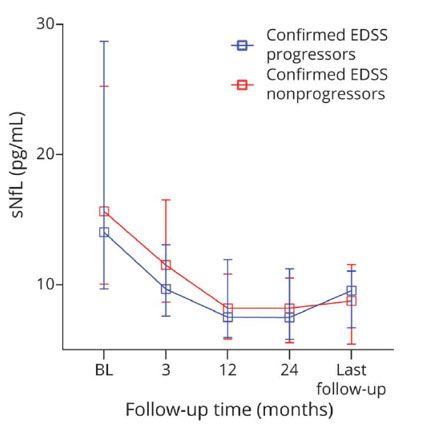
Figure 3: Longitudinal dynamics of NfL in those with disability progression (as measured by Expanded Disability Status Score, blue) and nonprogressors (red), all while under treatment with natalizumab and having no clinical relapses or MRI activity.[10]
Do MS disease modifying therapies impact NfL levels in RRMS?
Given the effect of MS disease modifying therapies on focal inflammation, it may not be surprising that therapies effective in reducing clinical relapses and new lesions in RRMS also reduce serum NfL levels.[[12], [13]] Currently, NfL is being explored as an outcome in proof-of-concept phase 2 trials of anti-inflammatory therapies in RRMS.
Do MS disease modifying therapies impact NfL levels in progressive MS?
Clinical trials of ocrelizumab in PPMS and siponimod in SPMS found robust and sustained reduction of serum NfL in association with treatment. These studies provided encouragement that NfL may be a useful outcome metrics for progressive MS trials. However, enthusiasm was reduced by the INFORMS trial of fingolimod in PPMS and ASCEND trial of natalizumab in SPMS. In both trials, the overall clinical outcome was negative but active treatment was associated with a robust reduction in NfL. Thus, NfL gave a “false-positive” result in those trials. Two other phase 2 trials in progressive MS found benefit on their primary MRI outcome, progression of brain atrophy, yet failed to show benefit on serum levels of NfL. [[2] [14]]. Thus, NfL gave a “false-negative” result in these two progressive MS trials: the trials met their primary and several secondary imaging outcomes, yet did not show a benefit on serum NfL levels. Accordingly, at this time, NfL does not appear to be a useful marker in progressive MS trials besides reflecting levels of focal inflammation (e.g. clinical relapses and new/active MRI lesions).
How can NfL be used in the routine monitoring of MS patients?
As described above, NfL can reflect the impact of focal inflammatory disease activity in MS and, thus, is an attractive blood biomarker for use in individual MS patient management. The exact role of serum NfL in the monitoring and management of MS patients is still being defined, but several important aspects of serum NfL need to be recognized when translating published group-based research findings into everyday individual-patient clinical practice: 1) the expected normal range of serum NfL is not well-defined and will depend upon the measurement assay and patient characteristics such as age, kidney function, and other medical conditions such as diabetes; 2) the kinetics of NfL need to be considered, including its increase 1 month or more after the onset of focal inflammation and its unknown clearance rate from the blood; 3) elevations in NfL are seen in some patients with new/active lesions but not all, making NfL an incomplete measure of the focal inflammation characterized by MRI; 4) aside from reflecting ongoing focal inflammation, NfL levels in progressive MS are not related to progression.
Nonetheless, MS providers are exploring how serum NfL might be utilized in routine patient management. It may be helpful to monitor as an overall trend of MS tissue injury. It may fill a monitoring role between MRIs, supplementing patient report and clinical exams. It is considerably less expensive than a brain MRI, so may be a useful supplement between routine MRIs, although its insensitivity relative to MRI needs to be recognized. At this time, serum NfL is not the sole basis of changes in long-term treatment regimens and does not replace MRI when evaluating whether new symptoms may represent active foal MS inflammation. Indeed, its delayed rise one month of more after the development of new focal lesions on MRI needs to be kept in mind.
Are there specific situations where serum NfL may be particularly useful?
There are specific situations where serum NfL may be particularly helpful in MS patient management.
- Monitor a patient after long-term disease modifying therapy has been stopped, keeping in mind various issues described above.
- Monitoring JCV seropositive who are receiving natalizumab therapy for potential progressive multifocal leukoencephalopathy, as reports have observed an increase in serum NfL prior to the clinical manifestations of PML. [15]
- Monitoring for disease activity in patients who are unable to have MRI (e.g. due to metal implants)
References
- Kuhle, J., et al., Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med, 2016. 54(10): p. 1655-61.
- Fox, R.J., et al., Neurofilament light chain in a phase 2 clinical trial of ibudilast in progressive multiple sclerosis. Mult Scler, 2021. 27(13): p. 2014-2022.
- Fox RJ, C.B., De Sèze J, Gold R, Hartung H-P, Jeffery D, Kappos L, Montalbán X, Weinstock-Guttman B, Singh C, Campbell N, Ho P-R, Su R, Engle B, Sangurdekar D, de Moor C, Fisher E, Kieseier BC, Rudick RA, Plavina T, Temporal Relationship of Serum Neurofilament Light (NfL) Levels and Radiological Disease Activity in MS Patients. ECTRIMS, 2018.
- Fitzgerald, K.C., et al., Contributors to Serum NfL Levels in People without Neurologic Disease. Annals of Neurology, 2022. 92(4): p. 688-698.
- Novakova, L., et al., Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology, 2017. 89(22): p. 2230-2237.
- Varhaug, K.N., et al., Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurology - Neuroimmunology Neuroinflammation, 2018. 5(1): p. e422.
- Kuhle, J., et al., Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology, 2019. 92(10): p. E1007-E1015.
- Disanto, G., et al., Serum Neurofilament Light: A Biomarker of Neuronal Damage in Multiple Sclerosis. Annals of Neurology, 2017. 81(6): p. 857-870.
- Filippi, P., et al., Neurofilament light chain and MRI volume parameters as markers of neurodegeneration in multiple sclerosis. Neuro Endocrinol Lett, 2020. 41(1): p. 17-26.
- Bridel, C., et al., Serum Neurofilament Light Association With Progression in Natalizumab-Treated Patients With Relapsing-Remitting Multiple Sclerosis. Neurology, 2021. 97(19): p. e1898-e1905.
- Gafson, A.R., et al., Serum Neurofilament Light and Multiple Sclerosis Progression Independent of Acute Inflammation. JAMA Network Open, 2022. 5(2): p. e2147588-e2147588.
- Disanto, G., et al., Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol, 2017. 81(6): p. 857-870.
- Novakova, L., et al., Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology, 2017. 89(22): p. 2230-2237.
- T. Williams, K.H., J. Nicholas, T. Katsanouli, H. Wellington, A. Heslegrave, H. Zetterberg, C. Frost, J. Chataway, Serum neurofilaments in secondary progressive multiple sclerosis: analysis from the ms-stat trial. ECTRIMS, 2020.
- Dalla Costa, G., et al., Serum neurofilaments increase at progressive multifocal leukoencephalopathy onset in natalizumab-treated multiple sclerosis patients. Annals of Neurology, 2019. 85(4): p. 606-610.