Q: How do we characterize progressive MS?
A: People with progressive MS have a disease course characterized by gradual worsening of neurologic disability independent of relapses (although symptom fluctuations, periods of stability, or superimposed relapses may occur)1. In people with primary progressive MS (PPMS), gradual worsening is present at disease onset while in secondary progressive MS (SPMS) a progressive course occurs following an initial relapsing course. The biological mechanisms that underlie SPMS and PPMS are considered largely the same (based on pathology, clinical and imaging features), and thus are both referred to as progressive MS (PMS).
Although the distinct classifications of relapsing-remitting MS (RRMS), SPMS and PPMS have historically been used2, growing evidence suggests that relapsing and progressive MS are not distinct entities, but rather gradual accumulation of disability independent of inflammatory disease activity can conceivably occur at any stage of the disease3. The pathological underpinnings of these two processes- focal inflammatory lesions with breakdown of the blood brain barrier for relapses and a combination of chronic processes (neurodegeneration, compartmentalized inflammation, failure of compensatory mechanisms) for gradual worsening of disability- are thought to vary throughout the disease course. Because both processes can occur simultaneously within a single patient and vary throughout the disease course, frequent assessments for the presence of disease activity and progression are needed. Thus, clinicians should make a retrospective determination (typically within the last 12 months) on 1) whether patients have disease activity (i.e. relapses, new/enlarging T2 lesion, or contrast-enhancing lesions on MRI) and 2) whether gradual worsening of disability (i.e. progression) has been present2. Of note, in a patient who is already on anti-inflammatory DMT and lacks disease activity, it can be difficult to know whether active inflammation would have been present without DMT use.
Despite the above concepts, DMTs are approved based on clinical trials in specific populations (relapsing forms of MS [clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive] and PPMS), and hence in this document we approach treatment with these categories in mind.
Q: How do we utilize DMTs in PPMS with and without disease activity?
A: There is only one medication approved by the FDA for people with PPMS (regardless of disease activity): ocrelizumab. Ocrelizumab is a monoclonal antibody which selectively depletes CD20+ B cells and is the primary treatment used for PPMS at the Mellen Center. In the ORATORIO trial, ocrelizumab reduced 12-week confirmed disability worsening (CDW) by expanded disability status scale (EDSS) and showed benefit in timed 25-foot walk (T25FW), MRI T2 lesion volume, and rate of brain atrophy compared to placebo in PPMS4. It is important to note that for participation in the ORATORIO trial patients had to be age 55 or younger and required the presence of oligoclonal bands (or elevated IgG index) in cerebrospinal fluid. This was animportant step forward in treatment, as previous randomized clinical trials (RCTs) of glatiramer acetate5, interferons (IFNs)6, and fingolimod7 failed to demonstrate efficacy in PPMS.
Subgroup analysis from the ORATORIO trial showed notable trends (p<0.3) for the benefit of ocrelizumab in younger patients (≤45), those with gadolinium-enhancing lesions at baseline, and males8. Therefore, at the Mellen Center, we use ocrelizumab for PPMS but recognize that younger patients, especially those with evidence of disease activity, are more likely to respond. Use should be carefully considered against potential risks in older patients or those without disease activity who are less likely to respond. Known risk factors for serious infection on anti-CD20 therapy include older age, higher levels of ambulatory disability, longer duration of therapy, prior exposure to immunosuppression, lymphopenia and hypogammaglobulinemia (IgG<500)9,10.
Rituximab (a chimeric monoclonal antibody directed at CD20) and ofatumumab (a fully humanized anti-CD20) are commonly used in relapsing forms of MS. Both rituximab and ofatumumab are sometimes used in PPMS extrapolating results from ocrelizumab, however obtaining insurance approval for these medications in PPMS is sometimes difficult.
Q: How do we utilize DMTs in people with SPMS with disease activity?
A: At the Mellen Center, DMTs are often offered to people with active SPMS (see definition above), with the goal of preventing the development of new and/or enlarging lesions, clinical relapse, and slowing disability progression. Although there have been limited trials specifically in the SPMS population, people with SPMS were included in many RRMS trials (given significant overlap between RRMS and SPMS). In the EXPAND trial12, siponimod reduced CDW and showed benefit on T2 lesion volume and percent brain volume change in people with SPMS. In the ASCEND trial13, natalizumab failed to show an effect on sustained CDW measured by T25FW or EDSS in SPMS but did show preservation of upper extremity function measured by 9 hole peg test (9HPT). The efficacy data for IFNs have had conflicting results across trials. A 1998 trial based out of Europe14 found delayed CDW measured by EDSS in people with SPMS treated with IFN beta 1b, while a 2004 North American trial15 did not. This may be related to a higher number of patients with active disease in the former study. Consistent with this idea, in combined analysis of the two trials, patients with more active disease were more likely to benefit from IFN beta 1b16.
Based on the trial results and concepts described above, in 2019 the FDA approved treatments already licensed for relapsing forms of MS also to treat SPMS with disease activity. The decision to initiate DMT should be made on a case-by-case basis, taking into consideration evidence of recent disease activity and weighing the risks versus benefits of DMT use. Special care should be made in patients who are older and may be at greater risk of complications10. However, when disease activity is present, DMT use should be considered.
At the Mellen Center, if a patient with SPMS develops a new demyelinating lesion while on DMT, we consider DMT escalation, or trial of DMT with a differing mechanism of action. Importantly, patients with SPMS who are older may have vascular or other co-morbidities, andtherefore, it is important to consider whether new lesions are related to vascular, or other non-demyelinating pathologies (including infections, particularly PML in patients on natalizumab).
Q: How do we utilize DMTs in people with SPMS without disease activity?
A: Current DMTs mainly target the (focal) inflammatory component of MS, and therefore the benefit of DMTs in people with SPMS without disease activity is less clear and many times is not recommended at the Mellen Center.
There is only one medication approved to treat SPMS by the FDA, independent of relapse activity. Mitoxantrone is indicated for reducing neurologic disability and/or the frequency of clinical relapses in patients with secondary progressive and progressive relapsing forms of MS. However, due to an adverse side effect profile (cardiotoxicity and hematologic malignancies) this medication is seldom used in clinical practice11.
Clinical trials of other DMTs specifically in people with SPMS have been performed, however, these trials included SPMS patients both with and without disease activity. Therefore, extrapolating the results to SPMS patients without disease activity is uncertain.
Whether to use medication approved for relapsing forms of SPMS in those without relapses or recent disease activity on MRI remains an area of significant debate. While clinical trial data do not fully support the use of these medications, sometimes clinicians at the Mellen Center do trial these therapies. Caution has to be taken as infectious risks with some of these medications may increase in the presence of more advanced disability9 and with greater age10, while benefit is less likely17. A common approach is to use these medications during a trial period (1-2years) and re-assess benefits (effect on disease progression, please see monitoring parameters below) and occurrence of adverse effects.
Q: How do we clinically monitor progressive MS?
A: Clinical monitoring in people with PMS should include surveillance for worsening disability as well as the presence of relapses. Monitoring for gradual worsening of disability in PMS should be conducted by neuroperformance measures, direct patient report, patient reported outcome (PRO) measures, and neurological examination. We evaluate patients approximately every 6-12 months with the following neuroperformance tests: Walking speed test (similar to the T25FW) to assess lower extremity function, manual dexterity test (similar to 9HPT) to assess upper extremity function, processing speed test (similar to Symbol Digit Modalities Test) to assess cognition, and low contrast letter acuity to assess vision. Meaningful thresholds of change for each of these variables are outlined below. PRO measures include the Neuro-QOL (Quality of Life in Neurological Disorders) assessment and patient determined disease steps (PDDS).
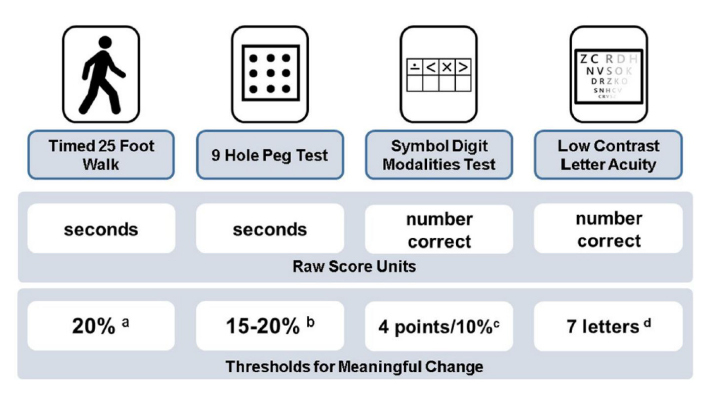
Figure 1. Multiple Sclerosis Functional Composite and suggested thresholds for meaningful change, as published in Ontaneda et al 201818.
a. Motl, RW, Cohen, JA, Benedict, R. Validity of the timed 25-foot walk as an ambulatory performance outcome measure for multiple sclerosis. Mult Scler 2017; 23: 704–710.
b. Feys, P, Lamers, I, Francis, G. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult Scler 2017; 23: 711–720.
c. Benedict, RH, DeLuca, J, Phillips, G. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler 2017; 23: 721–733.
d. Balcer, LJ, Raynowska, J, Nolan, R. Validity of low-contrast letter acuity as a visual performance outcome measure for multiple sclerosis. Mult Scler 2017; 23: 734–747.
The presence of accumulation of disability without focal inflammatory activity may raise a question about treatment initiation or escalation, however the data supporting this recommendation is weak. While in relapsing MS the treatment goal is for clinical stability, in PMS treatment goals are to either slow or in some cases even cease disability worsening. Assessment of the effect of treatment on disease progression can be challenging, as the rate of progression changes over time and is hard to predict. Creating realistic expectation of DMT treatment in PMS is important.
Q: How do we radiologically monitor progressive MS?
A: The pathological correlates of progression are rarely identified on conventional MRI. Although a number of imaging measures have been proposed to correlate with progressive disease (including whole brain atrophy, deep or gray matter atrophy, paramagnetic rim lesions, slowly evolving lesions, cortical lesions, and spinal cord area), they remain under investigation and are not currently used in routine clinical practice. However, there are multiple reasons to obtain routine imaging in people with progressive MS. These include 1) to monitor for the development of lesion activity in patients who are not on DMT, 2) to monitor the effectiveness of DMT, 3) to monitor for complications of DMT, and 4) to rule out other conditions that may mimic neurologic progression (such as myelopathy from cervical spondyloarthropathy).
For patients not on DMT and recently transitioned to SPMS we initially obtain brain MRI approximately yearly to assess for the development of lesions indicative of inflammatory activity, although this frequency can decrease once patients have been free of disease activity for at least 2-3 years. In some cases (when symptoms localize to the spinal cord), we also obtain spinal cord imaging to monitor for new lesion development or rule out alternative pathologies such as cervical spondyloarthropathy. If a new or enlarging demyelinating lesion indicative of inflammatory activity is seen on monitoring MRI, DMT initiation/reinitiation should be considered.
For patients who are on DMT and recently transitioned to SPMS, we monitor with brain MRI approximately yearly in order to monitor the efficacy of and potential (infectious) complications of DMT. In patients who are very stable, this frequency can decrease. If DMT is stopped, we obtain more frequent scans after discontinuation in order to monitor for the emergence of disease activity. Some clinicians at the Mellen Center also routinely monitor spinal cord MRI, particularly if symptoms localize to the spinal cord. If a new or enlarging demyelinating lesion indicative of focal inflammatory activity is seen on monitoring MRI, we consider DMT escalation, or trial of DMT with differing mechanism of action. As noted above, in patients on DMT who are older and at increased risk of complications, it is important to consider whether new brain lesions on MRI may be related to non-demyelinating (i.e. vascular, infectious) pathologies.
Q: When do we consider discontinuing DMTs in progressive MS?
A: The important question of when to discontinue DMT use in people with progressive MS remains unanswered. With age, MS disease activity often declines due to immunosenescence, and therefore the need for anti-inflammatory DMT use also may decline with age17. Furthermore, risk from some DMTs (including infection and malignancy) tend to increase with age and duration of DMT use9,10. There are two distinct scenarios to distinguish: A) whether an older patient who is clinically stable should continue on DMT, and B) whether an older patient with non-active progression should continue on DMT. For each scenario, the risk of disability from MS disease activity versus risks of treatment should be carefully weighed (ideally on an annual basis) in order to determine whether DMT use remains indicated. During this assessment, clinicians should consider specific DMT risks, patient age, disease duration, relapse history, and MRI-detected activity (e.g., frequency, severity, time since most recent relapse or gadolinium-enhanced lesion). AAN guidelines recommend that clinicians may advise discontinuation of DMT in people with SPMS who do not have ongoing relapses (or gadolinium-enhanced lesions on MRI activity) and have not been ambulatory (EDSS 7 or greater) for at least two years.19 As part of the discussion with patients, clinicians should also consider risk of worsening of neurological symptoms not well captured by the EDSS such as upper extremity function and cognition after DMT discontinuation. There currently are no published data from randomized clinical trials to provide guidance, however multiple trials on this topic are ongoing at the time this MCA was written (DISCOMS NCT03073603, STOP-I-EP NCT03653273, DOT-MS NCT04260711).
At the Mellen Center, we consider DMT discontinuation in people with progressive MS who are older than age 55 and who have had radiologic stability and no relapses for >5 years, based on meta-analysis of DMT efficacy by age17 and data from observational studies20,21. This mirrors the inclusion criteria for DISCOMS. Upon DMT discontinuation, patients should be monitored closely for return of disease activity with brain MRI initially every 6-12 months, and then yearly for several years. Frequency can be decreased once patients have been free of disease activity for at least 2-3 years. Some Mellen Center clinicians also obtain spinal cord imaging for monitoring purposes.
Q: What other counseling and management options are recommended for progressive MS?
A: When worsening disability is identified, a comprehensive rehabilitation strategy is needed, and multidisciplinary care with rehabilitation medicine, physical therapy, occupational therapy, and speech therapy (as needed) should be pursued. Other important disease considerations in PMS include assessment and treatment of co-morbidities that may quicken disease progression, including smoking, vascular co-morbidities, COPD, depression/anxiety, and obesity22. People with PMS should also be counseled on the benefits of exercise and the importance of smoking cessation. They should stay up-to-date on age-appropriate vaccinations, and be counseled on diet and wellness strategies. Symptom management is often extremely beneficial. Please see separate Mellen Center Approaches for further details on these topics.
Q: Do we recommend alternative/investigative pharmaceutical therapies in progressive MS?
A: There are a number of therapies currently under investigation for treatment of progressive MS. The data for these therapies, as of spring 2022, is summarized below.
High-dose simvastatin (80mg/day) was shown in a phase II trial to reduce rate of brain atrophy and showed statistically significant, although small, benefits in EDSS changes over a two-year time period23. Although further investigations are ongoing (MS-STAT-2, NCT03387670 ), it is sometimes considered for off-label use, however patients should be advised of the black-box warning of rhabdomyolysis. Lipoic acid (LA, 1200mg daily) has also been studied in a phase II clinical trial of people with SPMS and was found to slow progression of brain atrophy over 2 years compared to placebo control24. LA is an over-the-counter anti-oxidant which is relatively safe and well-tolerated, and can also be considered for off label-use. However, clinical benefits have not been proven and there was a suggestion of an increase in T2 lesion volume in the LA group, so further investigations are needed. BTK inhibitors, ibudilast, and mesenchymal stem cells are also being studied but are not clinically available (at the time this document was written) and require further investigation.
High-dose biotin was studied for treatment of progressive MS. Initial studies showed promise25. However, a large phase 3 clinical trial showed no benefit of high dose biotin but safety risks arising from interference of laboratory tests26. Therefore, at the Mellen Center, high-dose biotin is not recommended for treatment of progressive MS.
Approach last updated: March 24, 2022.
References
- Lublin FD, Coetzee T, Cohen JA, Marrie RA, Thompson AJ, MS on behalf of the IAC on CT in. The 2013 clinical course descriptors for multiple sclerosis. Neurology. 2020;94(24):1088 LP - 1092. doi:10.1212/WNL.0000000000009636.
- Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286. doi:10.1212/WNL.0000000000000560.
- Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials. JAMA Neurology. 2020;77(9):1132. doi:10.1001/jamaneurol.2020.1568.
- Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. New England Journal of Medicine. 2017;376(3):209-220. doi:10.1056/NEJMoa1606468.
- Wolinsky JS, Narayana PA, O’Connor P, et al. Glatiramer acetate in primary progressive multiple sclerosis: Results of a multinational, multicenter, double-blind, placebo-controlled trial. Annals of Neurology. 2007;61(1):14-24. doi:10.1002/ana.21079.
- Leary SM, Miller DH, Stevenson VL, Brex PA, Chard DT, Thompson AJ. Interferon -1a in primary progressive MS: An exploratory, randomized, controlled trial. Neurology. 2003;60(1):44-51. doi:10.1212/WNL.60.1.44.
- Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. The Lancet. 2016;387(10023):1075-1084. doi:10.1016/S0140-6736(15)01314-8.
- Wolinsky, JS, Montalban, X, Hauser, SL. Prespecified subgroup analyses of ocrelizumab efficacy in patients with primary progressive multiple sclerosis from the phase III ORATORIO study. Int J MS Care 2018; 20(Suppl 1): 1–128.
- Vollmer, B.L., Wallach, A.I., Corboy, J.R., Dubovskaya, K., Alvarez, E. and Kister, I. Serious safety events in rituximab-treated multiple sclerosis and related disorders. Ann Clin Transl Neurol. 2020;7: 1477-1487. https://doi.org/10.1002/acn3.51136.
- Schweitzer F, Laurent S, Fink GR, et al. Age and the risks of high-efficacy disease modifying drugs in multiple sclerosis. Curr Opin Neurol. 2019;32(3):305-312. doi:10.1097/WCO.0000000000000701.
- Martinelli Boneschi F, Vacchi L, Rovaris M, Capra R, Comi G. Mitoxantrone for multiple sclerosis. Cochrane Database of Systematic Reviews. Published online May 31, 2013. doi:10.1002/14651858.CD002127.pub3.
- Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. The Lancet. 2018;391(10127):1263-1273. doi:10.1016/S0140-6736(18)30475-6.
- R, Ho PR, Campbell N, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. The Lancet Neurology. 2018;17(5):405-415. doi:10.1016/S1474-4422(18)30069-3.
- Kappos L. Placebo-controlled multicentre randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. The Lancet. 1998;352(9139):1491-1497. doi:10.1016/S0140-6736(98)10039-9.
- MS TNASG on I beta 1b in SP. Interferon beta-1b in secondary progressive MS. Neurology. 2004;63(10):1788 LP - 1795. doi:10.1212/01.WNL.0000146958.77317.3E
- Kappos L, Weinshenker B, Pozzilli C, et al. Interferon beta-1b in secondary progressive MS: A combined analysis of the two trials. Neurology. 2004;63(10):1779 LP - 1787. doi:10.1212/01.WNL.0000145561.08973.4F.
- Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the Age-Dependent Efficacy of Multiple Sclerosis Treatments. Frontiers in Neurology. 2017;8. doi:10.3389/fneur.2017.00577.
- Ontaneda D, Cohen JA, Amato MP. Clinical outcome measures for progressive MS trials. Multiple Sclerosis Journal. 2017;23(12):1627-1635. doi:10.1177/1352458517729465.
- American Academy of Neurology. Practice Guideline Recommendations Summary: Disease-modifying Therapies for Adults with Multiple Sclerosis. American Academy of Neurology. Published 2018. Accessed February 27, 2018. https://www.aan.com/Guidelines/home/GuidelineDetail/898.
- Kister I, Spelman T, Patti F, et al. Predictors of relapse and disability progression in MS patients who discontinue disease-modifying therapy. Journal of the Neurological Sciences. 2018;391:72-76. doi:10.1016/j.jns.2018.06.001.
- Bsteh G, Hegen H, Riedl K, et al. Quantifying the risk of disease reactivation after interferon and glatiramer acetate discontinuation in multiple sclerosis: The VIAADISC score. European Journal of Neurology. 2021;28(5):1609-1616. doi:10.1111/ene.14705.
- Simmons SB, Schippling S, Giovannoni G, Ontaneda D. Predicting disability worsening in relapsing and progressive multiple sclerosis. Current Opinion in Neurology. 2021;34(3):312-321. doi:10.1097/WCO.0000000000000928.
- Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. The Lancet. 2014;383(9936). doi:10.1016/S0140-6736(13)62242-4.
- Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS. Neurology - Neuroimmunology Neuroinflammation. 2017;4(5):e374. doi:10.1212/NXI.0000000000000374.
- Tourbah A, Lebrun-Frenay C, Edan G, et al. MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: A randomised, double-blind, placebo-controlled study. Multiple Sclerosis Journal. 2016;22(13):1719-1731. doi:10.1177/1352458516667568.
- Cree BAC, Cutter G, Wolinsky JS, et al. Safety and efficacy of MD1003 (high-dose biotin) in patients with progressive multiple sclerosis (SPI2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Neurology. 2020;19(12):988-997. doi:10.1016/S1474-4422(20)30347-1.