What is the mechanism of action by which sphingosine 1-phosphate receptor (S1PR) modulators work?
Sphingosine kinases phosphorylate sphingolipids into the bioactive metabolite sphingosine 1-phosphate (S1P). While S1P mediates signaling via autocrine/paracrine mechanisms directly, its action via five high-affinity G protein-coupled receptors (sphingosine 1-phosphate receptors; S1PR1-5) is discussed here. As S1PRs are variably expressed systemically, their effects vary based the organ and cell-type, and raise the potential for off-target effects.
The main mechanism by which S1P modulators exert their effect with respect to multiple sclerosis is by binding to S1PR1. The S1PR modulator-receptor complex internalizes after binding on the lymphocyte, effectively antagonizing the receptor. This limits lymphocyte egress from lymph nodes, reducing the number of circulating lymphocytes in the peripheral blood, and thereby reducing their migration into the central nervous system (CNS). S1PR modulators can cross the blood-brain barrier and potentially exert direct CNS effects. Differences between S1PR modulators therapies is reviewed below.
How do the different therapies vary in their sphingosine 1-receptor selectivity?
|
Drug |
Sphingosine 1-receptor selectivity |
|
Fingolimod |
1,3,4,5 |
|
Siponimod |
1,5 |
|
Ozanimod |
1,5 |
|
Ponesimod |
1 |
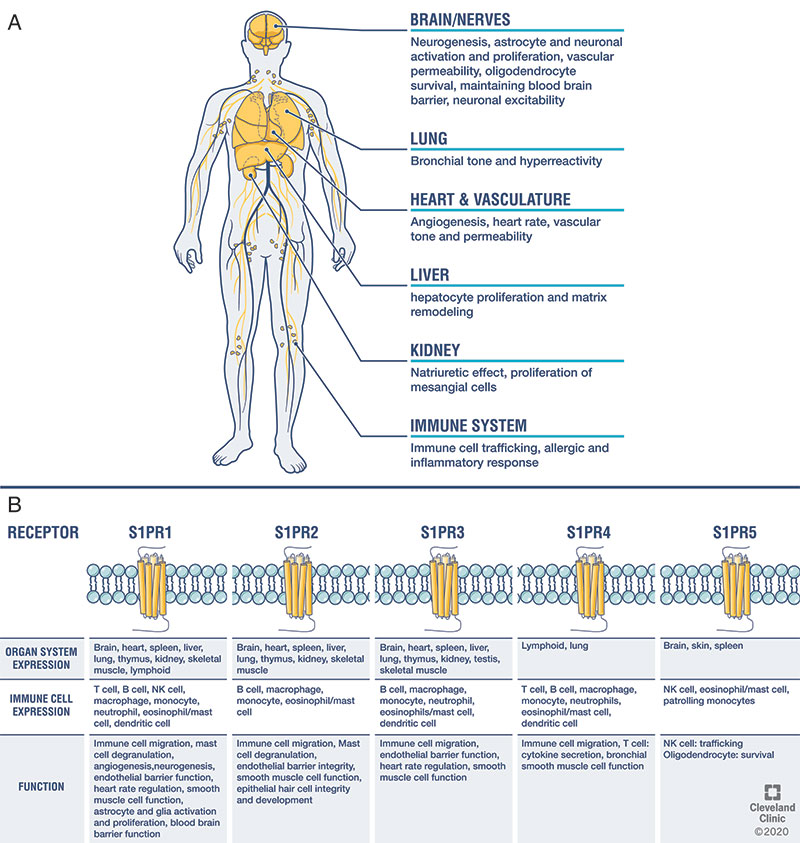
McGinley MP, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet. 2021 Sep 25;398(10306):1184-1194. Erratum in: Lancet. 2021 Sep 25;398(10306):1132. PMID: 34175020.1
What are the indications for these therapies?
These therapies are indicated for use in clinically isolated syndrome, relapsing remitting MS, and active secondary progressive MS. In relation to other available therapies for MS, they have moderate efficacy in preventing disease activity measured by the annualized relapse rate and MRI change (new/enlarging T2 lesions, brain and regional brain atrophy).
The only FDA approved S1PR modulator to date in pediatric MS is fingolimod.
|
Drug |
FDA approval in adults |
FDA approval in children |
|
Fingolimod |
9/2010 |
5/2018 10-18 years |
|
Siponimod |
3/2019 |
No |
|
Ozanimod |
3/2020 |
No |
|
Ponesimod |
3/2021 |
No |
What clinical trials have been performed for S1P modulators?
Their efficacy is thought to be comparable via the principal effects mediated by S1PR1 internalization. No head-to-head studies have been conducted between S1P modulators limiting the utility of cross-trial comparisons.
|
Drug |
Trials (n) |
Intervention |
ARR |
|
Fingolimod |
FREEDOMS Phase 3 FREEDOMS extension Phase 3 TRANSFORMS Phase 3 FREDOMSII Phase 3 INFORMS Phase 3 |
1.25/0.5mg/PBO 1.25/0.5mg 1.25/0.5mg/IFNβ1a 1.25/0.5mg/PBO 1.25mg/PBO |
0.18 vs 0.40
0.16 vs 0.33 |
|
Siponimod |
BOLD Phase 2 EXPAND Phase 3 |
#Adaptive dose* 0.25 to 2mg/PBO |
0.071 vs 0.160 |
|
Ozanimod |
RADIANCE Phase 2 RADIANCE Phase 3 SUNBEAM Phase 3 RADIANCE SUNBEAM extension Phase 3 |
1/0.5mg/PBO * ** 1 mg |
0.172 vs 0.276 0.181 vs 0.350 |
|
Ponesimod |
AC-058B201 Phase 2b AC-058B201 extension Phase 2 OPTIMUM Phase 3 |
40/20/10mg/PBO 40/20/10mg *** |
0.202 vs 0.290 |
ARR: annualized relapse rate; P2 – phase 2 (a,b where denoted); P3 – phase 3; PBO – placebo, PP – primary progressive MS, RR – relapsing remitting MS; SP – secondary progressive MS
#cohort 1: siponimod 10mg/2mg/0.5mg/PBO; cohort 2: siponimod 1.25mg/0.25mg/PBO
*part A: ozanimod 0.5mg/PBO injection; part B: ozanimod 1mg/PBO injection; part C: IFN β1a 30µg/PBO capsules
**part A: ozanimod 1 mg with PBO injection; part B ozanimod 0.5 mg with PBO injection; part C IFN β1a 30µg/PBO capsules
***Ponesimod 20 mg /teriflunomide 14 mg
What are significant drug-drug interactions?
For all S1P modulators, caution is recommended with concomitant use of:
- Anti-neoplastic, immunomodulating, noncorticosteroid immunosuppressive therapies
- Anti-arrhythmic drugs
- Drugs that decrease heart rate or atrioventricular conduction (eg. beta blockers or calcium channel blockers [diltiazem])
- QT-prolonging drugs
Specific interactions with hepatic enzyme metabolism are noted:
|
Drug |
Caution with concomitant use of medications that |
|
Fingolimod |
Inhibit/induce CYP4F2, induce 3A4 |
|
Siponimod |
inhibit both CYP2C9 and 3A4 (avoid); e.g. fluconazole inhibit 2C9 (caution) |
|
Ozanimod |
Inhibit CYP2C8 (e.g. gemfibrozil) Inhibit BCRP (e.g. cyclosporine, eltrombopag) Induce 2C8 (e.g. rifampin)* MAOIs (e.g. selegiline, phenelzine, linezolid) are contraindicated; wait 14 days between discontinuation of ozanimod and initiating MAOI; risk of hypertensive crisis. Increase norepinephrine or serotonin (opiates, SSRIs, SNRIs, TCAs, tyramine**); risk of hypertensive crisis. |
|
Ponesimod |
Induce multiple CYP450 (2J2, 3A4*, 3A5, 4F3A, 4F12) and UGT (1A1, 2B7) *CYP3A4 inducer (e.g. phenobarbital, phenytoin, carbamazepine, primidone, St. John’s Wort, glucocorticoids) UGT1A inducer |
MAOI – monoamine oxidase inhibitors
*CY2C8 inhibitors and inducers, see Appendix II of Ozanimod Mellen Center Approach
**tyramine rich foods see Appendix I of Ozanimod Mellen Center Approach
Concomitant use with medications that use the same hepatic enzyme systems may lead to greater or lesser systemic exposure of the S1P modulator. For example, concomitant use of a CYP3A4 inducer will lead to lower ponesimod levels.
What are contraindications for these therapies?
- Cardiac: myocardial infarction (MI) in the past 6 months, unstable angina, decompensated heart failure with hospitalization, class III/IV heart failure, baseline QTc ≥ 450 msec in males; ≥ 470 msec in females, arrhythmias requiring class Ia (e.g. quinidine, procainamide) or III anti-arrhythmics (e.g. amiodarone, sotalol).
History of Mobitz II 2nd degree or 3rd degree AV block, or sick sinus syndrome unless a pacemaker is functional.
Uncontrolled hypertension
- Severe untreated obstructed sleep apnea - ozanimod
- Concomitant use of a monoamine oxidase inhibitor - ozanimod
- Stroke/transient ischemic attack (TIA)
- CYP2C9*3/*3 genotype - siponimod
Which co-morbidities present an increased risk of adverse events with S1PR modulators?
- Cardiac/cerebrovascular: MI, arrhythmias, heart failure, hypertension, stroke/TIA
- Pulmonary: chronic obstructive pulmonary disease, asthma, pulmonary fibrosis
- Diabetes
- Uveitis
What testing is done before starting therapy?
Regardless of the S1PR modulator used, we recommend the following pretesting:
- Complete blood count (CBC) with differential
- Electrocardiogram (ECG)
- Liver function tests (LFTs)
- Varicella Zoster IgG antibody testing (VZV) and VZV vaccination if antibody-negative
- Discussion about human papilloma, influenza, and COVID-19 vaccination.
- Optical coherence tomography to screen for macular edema and repeat testing 3 months after initiation. Although optional for ozanimod per FDA recommendations unless at risk in patients with history of uveitis or macular edema, we recommend OCT or ophthalmological evaluation with all S1P modulators.
- CYP2C9 genotype testing with siponimod only; available at no cost through pharmaceutical company
- Baseline skin exam prior to or shortly after initiation; periodic exams in those with particular risk factors for skin cancer.
What are major adverse events reported of these therapies?
While fingolimod requires phosphorylation (by sphingosine kinase 1/2) to form its active metabolite, other S1PR modulators do not. The nonselective binding of fingolimod S1PRs confers a greater potential for adverse effects (e.g. bradycardia, hypertension, macular edema, reduced pulmonary function, and malignancy). The S1PR3 is expressed on vascular endothelium, further contributing to an added risk of hypertension and macular edema already possible with S1PR1 agnosim. However, the risk of these adverse effects exist in the S1PR modulator class and warrant awareness and appropriate monitoring for their occurrence.
Timing of transaminitis (ALT) can occur between 3 months (ponesimod, median) to 6 months (fingolimod [6-9 months majority], siponimod [majority], ozanimod [mean]). ALT levels typically return to normal in approximately 1 month after discontinuation with siponimod, ozanimod, and ponesimod while fingolimod can take 2 months. We recommend discontinuation if ALT exceeds 3X the upper limit of normal and closer interval of monitoring with any transaminitis, hyperbilirubinemia, or signs/symptoms of hepatic dysfunction.
Pulmonary assessment should be considered in patients who develop dyspnea or persistent cough.
Caution in the use of S1PR modulators in those with recurrent or chronic infections, particularly involving the urinary and upper respiratory tract, is recommended.
There is a risk of varicella-zoster reactivation and rare cases have been reported of malignancy due to oncogenic viruses and progressive multifocal leukoencephalopathy from the JC virus. Mitigation strategies include checking for varicella immunity and vaccination if titers are low/absent, cancer screenings per routine health guidelines, and surveillance brain imaging/prompt evaluation with concerning signs/symptoms.
|
Drug |
|
|
Fingolimod |
Macular edema (1.5% 1.25mg/0.5% 0.5mg/0.4% PBO) Hypertension (8% 0.5mg vs 4% PBO) Increased airway resistance (1.25 mg was associated with 5-fold increase in use of short-acting beta-agonists) QTc prolongation 14.0 msec (1.25 and 2.5 mg doses) Transaminitis (ALT > 5X ULN 4.5% vs 1% PBO) |
|
Siponimod |
Macular edema (1.8% vs 0.2% PBO) Hypertension (12.5% vs 9.2% PBO) Reduced FEV1 at 2 years (2.8% reduction compared to PBO) QTc prolongation 7.8 msec (2 mg dose) Transaminitis (ALT > 5X ULN 1.4% vs 0.5% PBO) |
|
Ozanimod |
Macular edema (0.3% vs 0.3% IFNβ1a) Hypertension (3.9% vs 2.1% IFNβ1a) Dose dependent reduction in FEV1 and FVC No QTc prolongation Transaminitis (ALT > 5X ULN 1.6% vs 1.3% IFNβ1a) |
|
Ponesimod |
Macular edema (1.1% vs 0% TER14mg) Hypertension (1.4% vs 0.2% TER) Reduced FEV1 (8.3% reduction at 2 years compared to 4.4 with TER 14mg) QTc prolongation 11.8 msec (40 mg) Transaminitis (ALT > 5X ULN 4.6% vs 2.5% TER) |
ALT – aminotransferase; PBO – placebo; TER – teriflunomide; FEV1 – forced expiratory volume in 1 second; FVC – forced vital capacity.
What monitoring is recommended while on treatment?
- OCT 3 months after initiation and repeat if any new blurred vision
- CBC with differential and liver function tests every 6 months
- BP regularly in patients with hypertension; adjust anti-hypertensive regimen as needed
- Cancer screening, including Papanicolaou (Pap) test, per routine guidelines
- Regular self-examination of skin every 3 months and annual full-body skin exam
- Annual MRI brain to evaluate for disease activity (determine therapeutic efficacy) and screen for adverse events (e.g. progressive multifocal leukoencephalopathy).
What are the recommendations for severe lymphopenia?
Consideration of alternative therapies should be considered if frequent infections occur with severe lymphopenia (< 300/µL).
How do the elimination pharmacokinetics differ?
|
Drug |
Elimination half-life |
Active metabolites |
|
Fingolimod |
6-9 days |
Yes |
|
Siponimod |
30 hours |
No |
|
Ozanimod |
21 hours; metabolites: CC112273 (10 days), CC1084037 (16 days) |
Yes |
|
Ponesimod |
33 hours |
No |
How does lymphocyte reduction compare?
|
Drug |
Lymphocyte reduction from baseline |
ALC nadir (cells/µL) |
Lymphocyte restoration after discontinuation |
Proportion of patients with ALC<0.2 x 109/L |
|
Fingolimoda |
70-80% |
500 |
6 weeks |
18% |
|
Siponimodb |
70-80% |
560 |
10 days |
NA |
|
Ozanimodc |
55% |
800 |
4-12 weeks |
3.3% |
|
Ponesimodd |
60-70% |
650 |
7 days |
3.2% |
Reference2
a. Fingolimod. Package insert. Novartis Pharmaceuticals Corporation; 2019.
b. Siponimod. Package insert. Novartis Pharmaceuticals Corporation; 2022.
c. Ozanimod. Package insert. Celgene Corporation, a Bristol Myers Squibb company; 2022.
d. Ponesimod. Package insert. Janssen Pharmaceuticals, Inc.; 2021.
ALC – absolute lymphocyte count
Is first-dose observation required?
|
Drug |
First dose observation requirement* |
|
Fingolimod |
Yes+ |
|
Siponimod |
Yes if pre-existing cardiac conditions#,+ |
|
Ozanimod |
No; consider if pre-existing cardiac conditions# |
|
Ponesimod |
No; consider if pre-existing cardiac conditions# |
*including reinitiation after discontinuation > 14 days and dose increases
+Observe for bradycardia for ≥6 hours; monitor heart rate (HR) and blood pressure (BP) hourly; electrocardiogram prior to initiation and end of observation period.
Monitor until resolution if:
- HR < 45 beats per minute (bpm) in adults; HR < 55 bpm 12-18 years, or HR < 60 bpm 10-12 years
- Atrioventricular block
- Lowest postdose HR is at the end of the observation period
- Symptomatic bradycardia with ECG until resolved
Continue overnight observation if intervention required; repeat first-dose monitoring for second dose.
Observe patients overnight if at higher risk of symptomatic bradycardia, heart block, prolonged QTc (>450 msec in males; >470 msec in females), or if taking drugs with known risk of torsades de pointes (e.g. class Ia – quinidine, procainamide; class III – amiodarone, sotalol).
#sinus bradycardia (HR < 55 bpm), first or second-degree (Mobitz type I) AV block, history of MI or heart failure
What are considerations for missed doses and re-initiation?
|
Drug |
0-2 weeks after initiation |
3-4 weeks after initiation |
> than 4 weeks after initiation |
|
Fingolimod |
FDO if missed 1 day of treatment |
FDO if missed ≥ 7 days |
FDO if missed > 14 days |
|
Siponimod |
Reinitiate titration per genotype indicated dosing if dose missed for > 24 hours during initiation |
Reinitiate titration per genotype indicated dosing if 4 or more consecutive daily doses are missed |
|
|
Ozanimod |
Reinitiate titration regimen if 1 dose missed |
Continue 0.92 mg daily if dose missed |
|
|
Ponesimod |
Reinitiate titration if 4 consecutive doses are missed |
Reinitiate titration if 4 or more consecutive daily doses are missed |
|
What are considerations for rebound disease activity after discontinuation and switching therapy?
Rebound disease activity may occur 2-4 months after discontinuation of S1PR modulators, particularly in patients with highly active MS prior to initiation3, 4. Efforts should be taken to gaps between switching therapies and informing patients of the risks associated with discontinuation5.
How are the different therapies dosed?
|
Drug |
Days |
|
Fingolimod |
0.5 mg daily |
|
Siponimod |
Wild-type CYP2C9 genotypes (*1/*1, *1/*2, *2/*2) 1-2: 0.25 mg 3: 0.50 mg 4: 0.75 mg 5: 1.25 mg 6 and thereafter: 2 mg daily Prescribed as 0.25 mg #12 pills followed by 2 mg pills.
CYP2C9 genotypes (*1/*3, *2/*3) 1-2: 0.25 mg 3: 0.50 mg 4: 0.75 mg 5 and thereafter: 1 mg daily Prescribed as 0.25 mg #7 pills followed by 1 mg pills. CYP2C9 genotype (*3/*3) Contraindicated |
|
Ozanimod |
1-4: 0.23 mg 5-7: 0.46 mg 8 and thereafter: 0.92 mg daily |
|
Ponesimod |
1-2: 2 mg 3-4: 3 mg 5-6: 4 mg 7: 5 mg 8: 6 mg 9: 7 mg 10: 8 mg 11: 9 mg 12-14: 10 mg 15 and thereafter: 20 mg daily |
Which are special considerations to vaccinations?
All age-appropriate vaccination including the annual influenza and COVID-19 vaccinations/boosters are recommended.
The Centers for Disease Control Advisory Committee on Immunization Practices also recommends human papilloma virus (HPV) vaccination for everyone through age 26 years if not adequately vaccinated when younger. HPV infections (papilloma, dysplasia, warts, and HPV-related cancer) have been reported with fingolimod in the postmarketing setting6. Vaccination against HPV should be considered prior to treatment initiation of all S1PR modulators.
Killed, inactivated, and mRNA vaccines are considered safe while live vaccines are not recommended.
Examples of live vaccines routinely used in the US include: mumps, measles rubella (MMR); varicella (Varivax), rotavirus, intra-nasal influenza. Non-routinely used live vaccines in the US are: adenovirus (military use), typhoid vaccine (Ty21a), smallpox/monkeypox (Jynneos), and Bacille Calmette-Guerin (BCG; used for bladder cancer treatment). Live vaccines that may be recommended for international travel include: yellow fever; tetravalent dengue; live attenuated oral polio; mumps, measles, rubella and varicella (MMRV); measles; mumps; rubella.
Delay starting therapy for 1 month after obtaining a live vaccine.
Delay the use of live-vaccines for the following periods after the last S1P modulator dose:
|
Drug |
Delay live vaccination |
|
Fingolimod |
2 months |
|
Siponimod |
4 weeks |
|
Ozanimod |
3 months |
|
Ponesimod |
1-2 weeks |
What are considerations during pregnancy?
We recommend that women of childbearing potential need effective contraception during treatment as S1P is important for vascular and neural development during embryogenesis.
Use of S1PR modulators is not expected to interact with the efficacy of oral contraceptives.
|
Drug |
Delay conception after discontinuing S1PR modulator |
|
Fingolimod |
2-3 months |
|
Siponimod |
14 days |
|
Ozanimod |
3 months |
|
Ponesimod |
1 week7 |
What are considerations during breastfeeding?
The therapies are secreted in breast milk in animal studies. We generally do not recommend their use during breastfeeding but risks/benefits should be reviewed on an individual basis.
What are considerations when selecting a S1PR-modulator?
The comparative efficacy between therapies is thought to be similar. Factors that influence selection include insurance coverage, safety profile, convenience of startup, and drug-drug interactions. Selection of a S1PR modulator can also be influenced by co-morbidities such as concurrent treatment for conditions such as ulcerative colitis and contraindication in individuals with poor cardiac function.
References
- McGinley MP, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet. 2021;398(10306):1184-94. Epub 20210624. doi: 10.1016/S0140-6736(21)00244-0. PubMed PMID: 34175020.
- Bravo GA, Cedeno RR, Casadevall MP, Ramio-Torrenta L. Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives. Cells. 2022;11(13). Epub 20220629. doi: 10.3390/cells11132058. PubMed PMID: 35805142; PMCID: PMC9265592.
- Litwin T, Smolinski L, Czlonkowka A. Substantial disease exacerbation in a patient with relapsing-remitting multiple sclerosis after withdrawal from siponimod. Neurol Neurochir Pol. 2018;52(1):98-101. Epub 20171013. doi: 10.1016/j.pjnns.2017.10.001. PubMed PMID: 29110882.
- Yoshii F, Moriya Y, Ohnuki T, Ryo M, Takahashi W. Neurological safety of fingolimod: An updated review. Clin Exp Neuroimmunol. 2017;8(3):233-43. Epub 20170618. doi: 10.1111/cen3.12397. PubMed PMID: 28932291; PMCID: PMC5575715.
- Rowles WM, Hsu WY, McPolin K, Li A, Merrill S, Guo CY, Green AJ, Gelfand JM, Bove RM. Transitioning From S1P Receptor Modulators to B Cell-Depleting Therapies in Multiple Sclerosis: Clinical, Radiographic, and Laboratory Data. Neurol Neuroimmunol Neuroinflamm. 2022;9(4). Epub 20220517. doi: 10.1212/NXI.0000000000001183. PubMed PMID: 35581005; PMCID: PMC9128034.
- Macaron G, Ontaneda D. Clinical commentary on "Warts and all: Fingolimod and unusual HPV associated lesions". Mult Scler. 2019;25(11):1550-2. Epub 20181114. doi: 10.1177/1352458518813109. PubMed PMID: 30427261.
- JanssenMD. Use of PONVORY in Pregnancy and in Females of Reproductive Potential [updated 9/19/22]. Available from: https://www.janssenmd.com/ponvory/special-populations/pregnancy/use-of-ponvory-in-pregnancy-and-in-females-of-reproductive-potential.
a. Fingolimod. Package insert. Novartis Pharmaceuticals Corporation; 2019.
b. Siponimod. Package insert. Novartis Pharmaceuticals Corporation; 2022.
c. Ozanimod. Package insert. Celgene Corporation, a Bristol Myers Squibb company; 2022.
d. Ponesimod. Package insert. Janssen Pharmaceuticals, Inc.; 2021.