Breast cancer is when breast cells mutate and become cancerous cells that multiply and form tumors. Breast cancer typically affects women age 50 and older, but it can also affect men, as well as younger women. Healthcare providers may treat breast cancer with surgery to remove tumors or treatment to kill cancerous cells.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
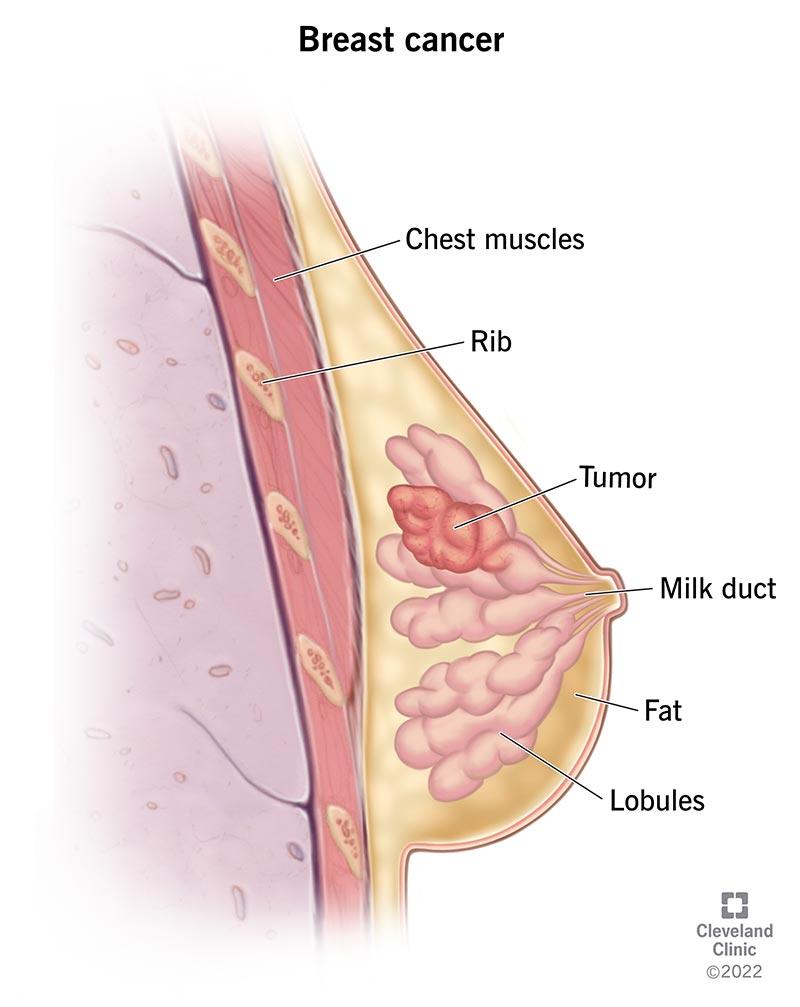
Image content: This image is available to view online.
View image online (https://my.clevelandclinic.org/-/scassets/Images/org/health/articles/3986-breast-cancer)
Breast cancer is one of the most common cancers that affects women. It happens when cancerous cells in your breasts multiply and become tumors. About 80% of breast cancer cases are invasive, meaning a tumor may spread from your breast to other areas of your body.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Breast cancer typically affects women age 50 and older, but it can also affect women who are younger than 50. Men may also develop breast cancer.
Healthcare providers determine cancer types and subtypes so they can tailor treatment to be as effective as possible with the fewest possible side effects. Common types of breast cancer include:
Less common breast cancer types include:
Advertisement
Healthcare providers classify breast cancer subtypes by receptor cell status. Receptors are protein molecules in or on cells’ surfaces. They can attract or attach to certain substances in your blood, including hormones like estrogen and progesterone. Estrogen and progesterone help cancerous cells to grow. Finding out if cancerous cells have estrogen or progesterone receptors helps healthcare providers plan breast cancer treatment.
Subtypes include:
Video content: This video is available to watch online.
View video online (https://cdnapisec.kaltura.com/p/2207941/sp/220794100/playManifest/entryId/1_laykbcij/flavorId/1_5f3sgelj/format/url/protocol/https/a.mp4)
Common signs of breast cancer and what to look for.
The condition can affect your breasts in different ways. Some breast cancer symptoms are very distinctive. Others may simply seem like areas of your breast that look very different from any other area. Breast cancer may not cause noticeable symptoms either. But when it does, symptoms may include:
Experts know breast cancer happens when breast cells mutate and become cancerous cells that divide and multiply to create tumors. They aren’t sure what triggers that change. However, research shows there are several risk factors that may increase your chances of developing breast cancer. These include:
Advertisement
The most significant complication is metastatic breast cancer — breast cancer that spreads to other areas of your body, including your brain, bones, liver and lungs. Studies show about 1 in 3 women who have early-stage cancer later develop metastatic breast cancer.
Healthcare providers may do physical examinations or order mammograms to check for signs of breast cancer. But they do the following tests to diagnose the disease:
Healthcare providers use cancer staging systems to plan treatment. Staging cancer also helps providers set a prognosis, or what you can expect after treatment. Breast cancer staging depends on factors like breast cancer type, tumor size and location, and whether cancer has spread to other areas of your body. Breast cancer stages are:
Advertisement
Surgery is the primary breast cancer treatment, but healthcare providers may use other treatments. Breast cancer surgeries include:
Providers may combine surgery with one or more of the following treatments:
Common chemotherapy and radiation therapy side effects include fatigue, nausea and vomiting. Targeted therapy, immunotherapy and hormone therapy have similar side effects, including gastrointestinal issues like constipation and diarrhea.
People react differently to breast cancer treatments. If you’re receiving treatment, ask your healthcare provider how treatment may affect you, including how it may affect your daily life. Also, ask your provider about palliative care. Palliative care helps manage breast cancer symptoms and treatment side effects so you’re as comfortable as possible as you go through treatment.
All surgeries have potential complications, and breast cancer surgery is no exception. As you’re considering your options, it’s important to remember that surgery removes potentially life-threatening cancer. In general, the risks of breast cancer outweigh the complications.
Advertisement
If you’re having breast cancer surgery, ask your healthcare provider to explain potential complications, which may include:
Breast cancer survival rates vary based on several factors, like whether the cancer is invasive or noninvasive, the cancer type and the cancer stage. According to data kept by the National Cancer Institute (U.S.), overall, 91% of people with breast cancer were alive five years after diagnosis. The institute organizes breast cancer survival rates by stages:
| Breast cancer stage | Five-year survival rate |
|---|---|
| Local | 99% |
| Regional | 86% |
| Distant | 30% |
| Breast cancer stage | |
| Local | |
| Five-year survival rate | |
| 99% | |
| Regional | |
| Five-year survival rate | |
| 86% | |
| Distant | |
| Five-year survival rate | |
| 30% |
As you think about breast cancer survival rates, remember, they’re only estimates based on other people’s experiences. Cancer affects different people in different ways. If you have specific questions about cancer survival rates, talk to your healthcare provider. They’re your best resource because they know your situation.
Right now, more people are being diagnosed with early-stage breast cancer — meaning they’re diagnosed when it’s easier to treat — and fewer people are dying of breast cancer.
Data shows 99% of people with early-stage breast cancer were alive five years after diagnosis. In some cases, they may be considered cured of breast cancer. But breast cancer can come back, and when it does, it may come back as metastatic breast cancer.
Outlook may also depend on race. According to the American Cancer Society, Black women are slightly less likely to develop breast cancer than white women. But Black women are more likely to die of breast cancer than white women.
You may not be able to prevent breast cancer. But you can reduce your risk of developing it. Just as important, regular self-exams and mammograms can help detect breast cancer early on, when it’s easier to treat.
There’s no sure way to reduce breast cancer risk, but the American Cancer Society (ACS) has the following advice for all females:
Some women have an increased risk for breast cancer because family members have it or they inherited a genetic mutation. If that’s your situation, you may want to consider the following:
Living with breast cancer may not be easy. You may have days when you feel overwhelmed by your situation. Consider the following suggestions for taking care of yourself as you go through breast cancer diagnosis and treatment:
Contact your provider if your symptoms seem to be getting worse or if you have new symptoms, like pain or weakness in a different part of your body.
You should go to the emergency room if your reaction to cancer treatment is stronger than you expected. For example, you should go to the emergency room if you’re severely dehydrated from constant vomiting.
Most people have lots of questions when they first learn they have breast cancer. Here are some ideas of questions you may want to ask your provider:
You can have breast cancer for years before noticing changes in your breasts like a lump. That said, not all lumps or bumps are cancer. Check with a healthcare provider if you have an unusual bump or mass that doesn’t go away after a few days.
That depends on several factors, including the type of breast cancer you have, whether it’s hereditary and, the tumor stage and grade. If you have breast cancer, ask your healthcare provider for information about what you can expect.
Yes, men can get breast cancer, but it’s not common. Approximately 2,600 men develop male breast cancer every year in the United States, making up less than 1% of all cases.
In 2023, 1 in 8 women in the United States will learn they have breast cancer. If you’re among those 1 in 8, there are a few more numbers you may want to consider. Nearly 4 million people in the United States are breast cancer survivors. Data show that overall, 91% of people with breast cancer were alive five years after diagnosis. And many experts are actively gathering information that may help providers tailor breast cancer treatment.
You can count on your healthcare team to continually evaluate newly approved treatments that you might be able to use. And here’s something else: If you have breast cancer, you can count on your team to be there for you, from diagnosis onward.

Sign up for our Health Essentials emails for expert guidance on nutrition, fitness, sleep, skin care and more.
Learn more about the Health Library and our editorial process.
Cleveland Clinic’s health articles are based on evidence-backed information and review by medical professionals to ensure accuracy, reliability and up-to-date clinical standards.
Cleveland Clinic’s health articles are based on evidence-backed information and review by medical professionals to ensure accuracy, reliability and up-to-date clinical standards.
A breast cancer diagnosis can turn your world upside down. At Cleveland Clinic, we offer expertise, compassion and personalized treatment plans.
