TAVR is a procedure to replace your heart’s aortic valve. It’s an alternative to open-heart surgery. TAVR treats a narrowed aortic valve (aortic stenosis), which can overwork your heart and increase your risk of serious complications. TAVR gives you a new valve so you can feel better and protect your heart.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
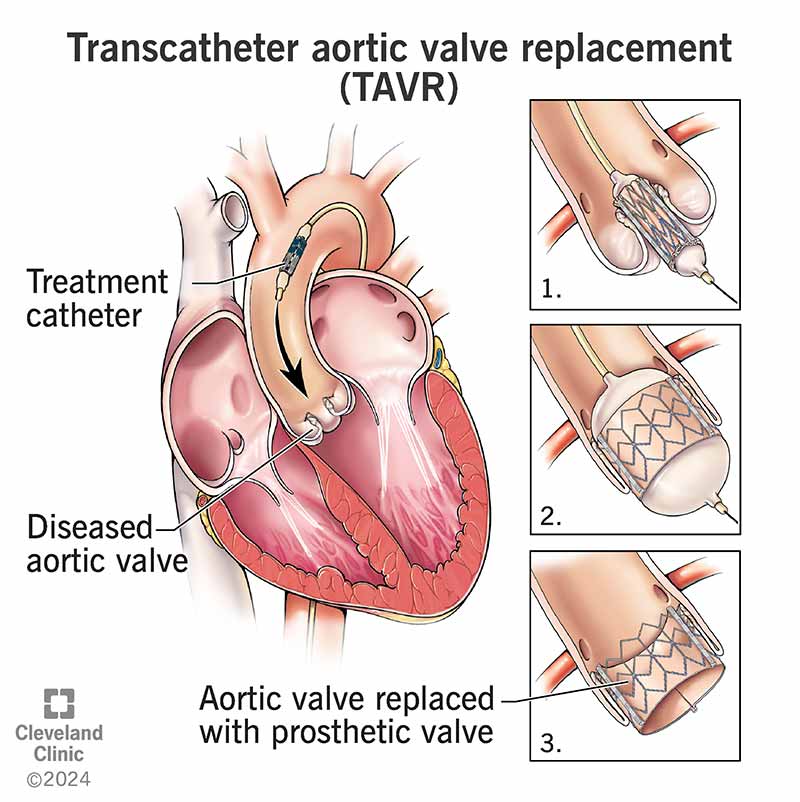
Image content: This image is available to view online.
View image online (https://my.clevelandclinic.org/-/scassets/images/org/health/articles/TAVR-transcatheter-aortic-valve-replacement)
TAVR (transcatheter aortic valve replacement) is a minimally invasive procedure that replaces your aortic valve without open-heart surgery. An interventional cardiologist inserts a thin tube (catheter) into an artery, typically in your groin. They guide the catheter to your heart. The catheter delivers your new valve, placing it inside your old one.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Receiving a new aortic valve can help you feel better and live longer. It can also protect your heart. That’s because your heart pumps blood through your aortic valve and out to your entire body. If the valve isn’t working right, your heart is forced to work harder to push blood through. This extra workload can damage your heart over time, leading to heart failure.
Transcatheter aortic valve implantation (TAVI) is another name for TAVR.
TAVR treats aortic stenosis. This is the narrowing of your aortic valve or the area around it. Narrowing usually happens because of:
You may need to fast (no food or drinks other than water) and stop taking certain medications before having TAVR. Your healthcare provider will tell you exactly what to do. Follow their guidance and ask if there’s anything you don’t understand.
Your provider may also run some tests, including blood and imaging tests, to help plan the procedure.
At the start of the procedure, you’ll receive sedation and/or anesthesia. You’ll also receive medicine to prevent blood clots.
Advertisement
To perform TAVR, an interventional cardiologist will:
TAVR typically takes about an hour or two from start to finish.
Transcatheter aortic valve replacement has several advantages over surgery. These include:
Many people who need aortic valve replacement are “high-risk” because of severe stenosis or other health conditions. That means they have a greater risk of complications or death from open-heart surgery. TAVR is an excellent alternative for them.
Despite this, some people may still benefit from an aortic valve replacement surgery. Talk to your healthcare provider about what’s right for you.
With advancements in valve and catheter technology, TAVR complications don’t happen often. But when problems do arise, they may include:
TAVR has many benefits. But it’s not right for everyone. The most common reasons your provider may not recommend TAVR include:
After you wake up from surgery, you’ll need to stay in bed for at least several hours. This is to prevent bleeding from the spot where the catheter went in. Your care team will make sure the stitches are secure before you get up.
Advertisement
A provider may prescribe medicine to keep you from getting an infection or blood clot after the procedure.
Most people who have transcatheter aortic valve replacement can leave the hospital within one day. Some may stay two days or more. It depends on how your procedure went and your overall health.
Your healthcare provider will explain how long your recovery is likely to take. In general, most people can return to most of their normal activities within days. For some, it may take a little longer.
You’ll need to wait at least one week before moving heavy items or doing other physical activity. You can typically go back to work two weeks after TAVR and drive after one month. Complete recovery takes six to 10 weeks.
Your healthcare provider will likely refer you to a cardiac rehab program. This is a prescribed workout plan that involves a team of providers from several different fields. You’ll start rehab within several days of your TAVR procedure to increase your heart’s strength and endurance.
Your healthcare provider will tell you when you should see them for follow-up care. You can expect a checkup a month after your TAVR procedure and then once a year after that. Regular follow-up care is vital. It allows your provider to check your heart and see how you’re feeling. They may also run tests to make sure your new valve is working well.
Advertisement
After TAVR, contact your provider if you have:
Someone’s going to put a new valve into your heart? That can feel scary. But keep in mind that you’re not the first person who’s had this procedure. Thousands of people have had TAVR. You can also give yourself some peace of mind by choosing a provider with a lot of experience performing this procedure.
Advertisement
Learn more about the Health Library and our editorial process.
Cleveland Clinic's health articles are based on evidence-backed information and review by medical professionals to ensure accuracy, reliability, and up-to-date clinical standards.
Cleveland Clinic's health articles are based on evidence-backed information and review by medical professionals to ensure accuracy, reliability, and up-to-date clinical standards.
It can be scary and overwhelming when something is happening with your heart valves. Cleveland Clinic heart specialists are ready to get you the help you need.
