Multiple sclerosis (MS) damages the protective cover around nerves called myelin in your central nervous system. It can cause muscle weakness, vision changes, numbness and memory issues. While there isn’t a cure, treatment options can help you manage symptoms and slow disease progression.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Video content: This video is available to watch online.
View video online (https://cdnapisec.kaltura.com/p/2207941/sp/220794100/playManifest/entryId/1_kuhfprit/flavorId/1_5f3sgelj/format/url/protocol/https/a.mp4)
Learn more about multiple sclerosis from Marisa McGinley, DO.
Multiple sclerosis (MS) is an autoimmune condition that affects your brain and spinal cord (central nervous system).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
With MS, your immune system mistakenly attacks myelin cells. These are the protective covers (sheaths) that surround your brain and spinal cord nerves. Myelin sheath damage interrupts messages (signals) that your nerves send throughout your body to perform functions like vision, sensation and movement.
Myelin damage can occur in your brain, spinal cord and nerves that supply your eyes. There’s no cure for MS, but treatment is available to help minimize ongoing damage and help you manage symptoms.
There are four types of multiple sclerosis. You can think of the types as a way for your provider to describe your symptoms, instead of being four different conditions:
Advertisement
Three rare MS variants include:
Studies show that there are almost 1 million adults in the U.S. living with multiple sclerosis.
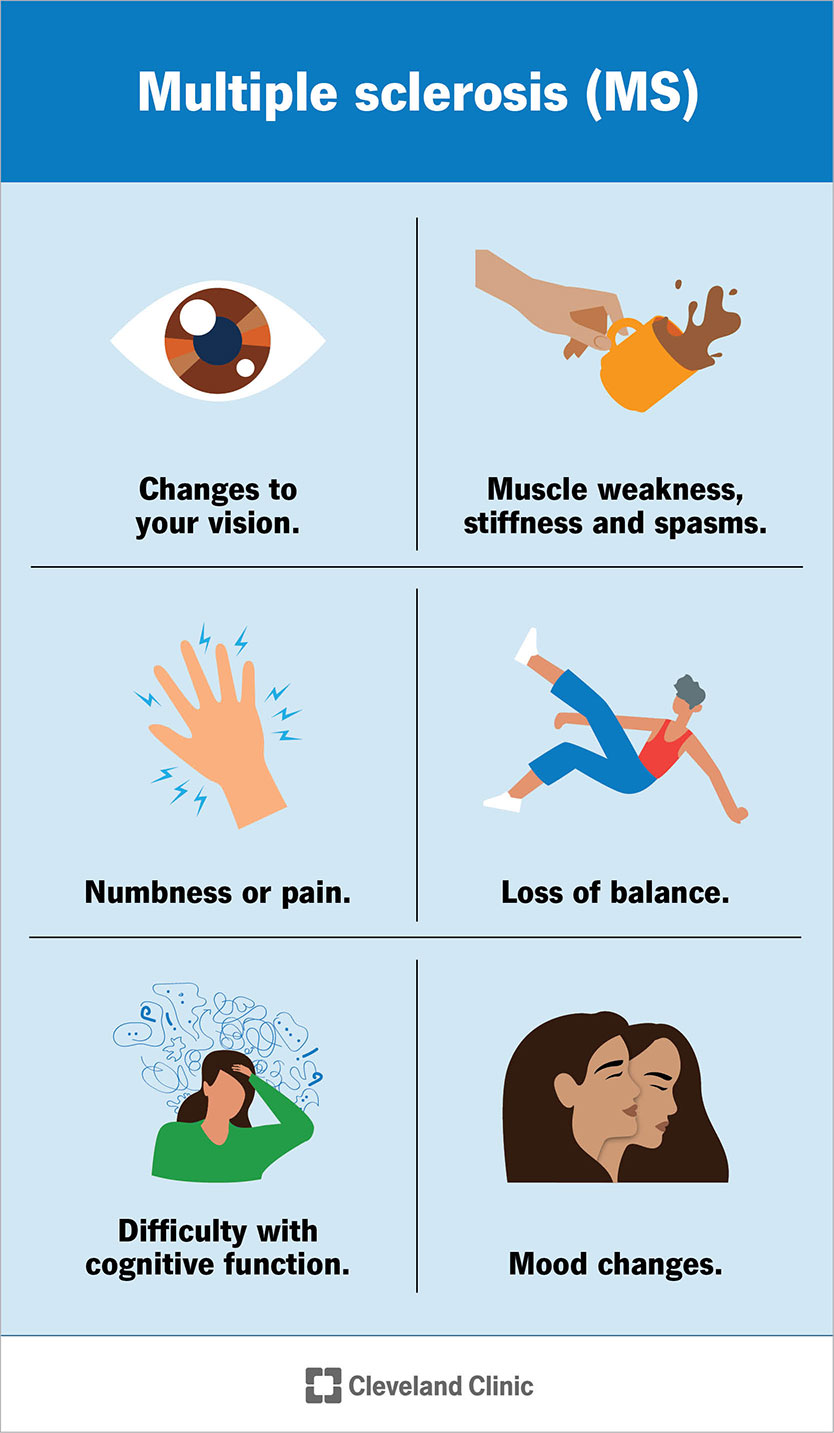
Image content: This image is available to view online.
View image online (https://my.clevelandclinic.org/-/scassets/images/org/health/articles/17248-multiple-sclerosis)
Early signs and symptoms of MS include:
Common symptoms of MS include:
These symptoms vary from person to person and may fluctuate in severity from one day to the next. You may have a few of these symptoms, but it’s unlikely you’ll experience all of them at once.
This can be challenging to predict because everyone perceives “normal” in their own way. With MS, you may have periods of remission where your symptoms go away, and you feel more like yourself. You might even forget you have MS until symptoms flare up (return) again. This feeling of normalcy, and the degree of normalcy, can vary by type and stage.
Demyelination, or the destruction of myelin, causes multiple sclerosis. Myelin is a protective cover (sheath) around nerve cells (neurons) in your brain and spinal cord. It moves messages (signals) between your brain and the rest of your body to control functions like vision, sensation and movement.
Your immune system’s job is to protect your body from things that can harm it, like bacteria or viruses. With MS, your immune system becomes overactive and mistakes healthy myelin (and sometimes, the nerve cells below the myelin) as a threat to your body. Your immune system’s attack on the healthy myelin damages it. This is demyelination.
On an imaging test (an MRI), your provider can find evidence of myelin damage. They may refer to it as a scar, lesion or plaque. Messages don’t pass between nerve cells easily where there is myelin damage, which leads to the development of MS symptoms.
Advertisement
Experts aren’t sure why some people develop MS. Research suggests the following may contribute to an elevated risk of developing MS:
You may be more at risk of MS if you:
MS can affect anyone. Rarer cases can affect children.
Worsening or progressive symptoms of MS may lead to complications such as:
There isn’t a single diagnostic tool available to pinpoint the condition. Instead, a provider will diagnose MS after a physical exam, a neurological exam and testing.
During an exam, your provider will learn more about your symptoms and medical history. Testing may include blood work, MRIs of your brain and spinal cord, and an analysis of your spinal fluid.
It can take time before you receive an official MS diagnosis. You may need to make several trips to see your provider before you know for sure. This happens because MS symptoms can look like or happen with several other common conditions. While the delay in an official diagnosis can be frustrating, getting the right diagnosis helps your provider accurately treat your symptoms.
Advertisement
Diagnostic testing helps your provider rule out conditions with similar symptoms to MS. Testing may include:
If your primary care provider suspects you may have MS, they may refer you to see a neurologist. A neurologist is a doctor who specializes in treating conditions that affect the nervous system, which includes your brain and spinal cord.
There isn’t currently a cure for MS.
Multiple sclerosis treatment focuses on minimizing further damage, managing symptoms and preventing complications. Your treatment plan may include:
Other types of symptom management vary based on how the condition affects you. Management may include:
Advertisement
Your healthcare provider may recommend plasma exchange (plasmapheresis) if your body doesn’t respond well to certain medications during an MS attack. This is more effective in minimizing damage from an ongoing attack as opposed to preventing additional attacks in the long term.
Your provider can also discuss if any clinical trials are available to participate in. Clinical trials are tests of new medications or uses of existing medications on humans to find new treatment options for MS and other conditions.
Medications for multiple sclerosis can reduce relapses (periods when symptoms worsen or new symptoms develop) and the development of new lesions/scars, and slow the disease’s progression. Common types of medications for MS include:
DMTs for MS
Common disease-modifying therapies (DMTs) for MS and their administration types include:
Multiple sclerosis is a lifelong condition without a cure. However, available treatment options are very effective in helping manage symptoms and minimizing the frequency of flare-ups. Regardless of treatment, MS can lead to disability and make it difficult to do routine things without assistance over time. Your care team is available to help you throughout your MS journey, to take steps to prevent complications and improve your quality of life.
You can expect to have a normal life expectancy with MS. Older studies have shown that MS can take up to 10 years off of your life expectancy, but advances in treatment options have significantly improved this outlook. Only in very rare cases is MS fatal.
There isn’t a known way to prevent MS.
Disease-modifying therapies are the most effective way to reduce the number of flare-ups (also called relapses or attacks) you experience.
Leading a healthy lifestyle is also important. The choices you make can help slow disease progression. Your provider may recommend the following to stay healthy:
Coping with a chronic condition can be emotionally challenging. MS can sometimes affect your mood and memory. Working with a neuropsychologist or a mental health provider is an essential part of managing the condition long term.
Yes. MS can be a challenging condition to diagnose and manage, but your care team will help you every step of the way. Despite having a condition without a cure, you can still lead a fulfilling and active life with MS. Support is available to help you maximize your function both physically and mentally, from medications to therapy. There are even support groups you can join to help you connect with people who share a similar experience.
You should contact a healthcare provider if you experience the following:
Let your healthcare provider know if you have MS and experience new or worsening symptoms.
You may want to ask your healthcare provider:
Multiple sclerosis (MS) is a disruptive condition. Symptoms can flare up or get worse without any notice. MS can also make you more likely to injure yourself if you lose balance. A healthcare provider can help you manage this condition so you can get back to your routine safely. You may need to adapt your lifestyle as the condition progresses, like using mobility devices or wearing glasses. But most people with MS lead full and active lives with the support of their care team. Let your providers know if you have any questions about your treatment options or what symptoms or complications to look out for.

Sign up for our Health Essentials emails for expert guidance on nutrition, fitness, sleep, skin care and more.
Learn more about the Health Library and our editorial process.
Cleveland Clinic’s health articles are based on evidence-backed information and review by medical professionals to ensure accuracy, reliability and up-to-date clinical standards.
Cleveland Clinic’s health articles are based on evidence-backed information and review by medical professionals to ensure accuracy, reliability and up-to-date clinical standards.
Living with multiple sclerosis is different for everyone. Cleveland Clinic MS specialists are experts at diagnosing and treating this condition.
