Deep brain stimulation (DBS) is a treatment that involves an implanted device that delivers an electrical current directly to areas of your brain. That current improves how well those parts work. It’s most often used for conditions like Parkinson’s disease and epilepsy, but researchers are investigating if it can help many other conditions too.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
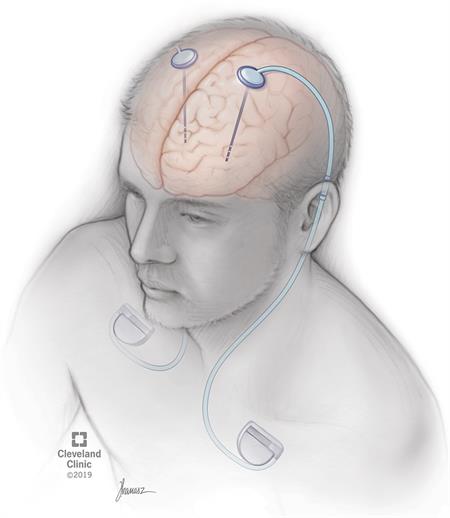
Deep brain stimulation (DBS) is a medical procedure that involves a mild electrical current delivered to a specific part of your brain. The electricity in that current stimulates the brain cells in that area, which can help several conditions. The current reaches your brain through one or more wires attached to a small device implanted underneath your skin near your collarbone.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
DBS treats conditions that affect how your neurons — a key type of brain cell — do their job. When neurons aren't working properly, that affects the abilities those neurons control. Depending on how severe the problem is, they can either partly or completely lose those abilities.
There are billions of neurons in each human brain, and these cells communicate with each other using electrical and chemical signals. Several brain conditions can make neurons in different parts of your brain less active. When that happens, those parts of your brain don’t work as well. Depending on the part of the brain affected, you can have disruptions in the abilities controlled in that area.
DBS uses an artificial electrical current to make those neurons more active, which can help with the symptoms of several different brain conditions. However, researchers still don’t know exactly how or why this works.
DBS can treat several conditions that affect your brain, including movement disorders, mental health conditions and epilepsy.
DBS has approval from the U.S. Food and Drug Administration to treat the following conditions:
Advertisement
Researchers are also looking into whether or not DBS can help with other conditions. Conditions that might benefit from DBS include:
It's important to keep in mind that while the above conditions might benefit from DBS, experts still don't know if this is the case. It usually takes years of research and clinical trials to determine if a medical procedure like DBS is helpful for conditions like these. While researchers are looking into them, DBS surgery to treat these conditions is not common.
As of 2019, experts estimate that about 160,000 people have had a procedure to implant a DBS device since the 1980s. Experts also estimate that about 12,000 procedures happen each year.
Before this procedure, your healthcare provider will discuss the advantages and disadvantages of having a DBS device implanted. They’ll also explain the possible risks that come with this surgery. They’ll also verify that you can have this surgery, which can involve other imaging scans or lab tests to look for any reasons you may not be able to have the procedure.
If you still decide you want to have the DBS implanted, your provider will then have you get detailed magnetic resonance imaging (MRI) and computed tomography (CT) scans of your brain. These scans will help your provider decide which location is the best place to place the wires for the DBS.
Before the procedure, your provider will also talk to you about the following:
Advertisement
This procedure actually involves two to three surgeries that usually happen at different times. The first one or two procedures are to insert the stimulation leads into each side of your brain at the same or separate times. The second procedure is to implant the stimulator battery — known as a pulse generator — under the skin of your upper chest.
Before these surgeries happen, your healthcare provider will usually insert an intravenous (IV) line to give you IV fluids. An IV also allows them to give you medications during the procedure as needed.
This procedure usually starts with your healthcare provider shaving the hair on your scalp. This makes it easier to place your head into a special frame that will hold your head still. The frame is set with four pins in your skull. This is done while you’re under sedation, and you likely won’t remember this part.
Once the frame is set, they’ll bring in an intra-operative CT scanner to take images of your brain and identify the trajectory used for the electrode placement. Once the CT scan is complete, the entry point is identified, sedation is turned back on and your head is cleaned with surgical prep. Local anesthetic is then injected to numb that area of your scalp and skull. Your neurosurgeon will then make a small cut (incision).
Advertisement
After making the incision, they'll use a surgical drill to make a small opening in your skull to insert the leads. Depending on the reason for surgery, you may be woken up for awake testing. This is mainly done for movement disorders. If awake, you may feel the vibrations or hear the sounds of drilling, but you shouldn’t feel pain from it. Your brain also can’t feel pain directly, so you won’t feel any pain as the neurosurgeon inserts the leads.
While the neurosurgeon places the leads, they’ll have you answer questions, read or look at pictures, or move your arms, legs, hands and feet in certain ways. This helps them be sure the leads are in the right place. They'll also do another CT scan to ensure the lead placement is accurate.
The number of leads and their placement depend on your case. Some people may only have one lead, while others may have multiple leads on one or both sides of their heads. Once the electrodes are secure, the ends of the electrodes are protected with a plastic cap and tunneled under your skin to the back of your head. The incisions are then cleaned and closed.
You’ll go to recovery where a CT scan is done to confirm placement of the electrode and to make sure there’s no blood. You’ll spend one night in the hospital for observation, and most people go home the next day.
Advertisement
The second procedure, surgery to implant the pulse generator, usually happens at a later date. This procedure involves general anesthesia, which means you'll be asleep so you won't feel any pain or discomfort during the surgery.
Your surgeon will make a small incision in your skin just below your collarbone during this procedure. They'll then create a small pouch-like space under your skin to hold the pulse generator. They'll then insert an extension wire that travels between the outside of your skull and the underside of your skin.
They'll make the wire travel downward until the far end is underneath your skin near your collarbone at the pocket for the battery. They'll then connect the extension wires to the DBS electrodes and the other end of the extension wire to the pulse generator. It's then placed into the pouch-like space under your skin before sewing it shut. You’ll go home the same day.
Your healthcare provider will schedule a follow-up appointment that will take place within a few weeks of the pulse generator implantation procedure. At this appointment, they'll start programming the pulse generator.
All pulse generators now in use have a wireless antenna built-in. That allows your healthcare provider to access and program the device from outside your body. Finding the right settings for the pulse generator may take some time and additional visits for adjustments.
Most pulse generators have special batteries that have long lifespans. Standard batteries for these devices last about three to five years. Some devices use rechargeable batteries, which can last about nine years. Replacing the battery also takes a surgery procedure, but this is usually shorter and quicker than the original surgery to implant the pulse generator. You'll still go home the same day for battery replacements.
DBS has several advantages. These include:
Because DBS does involve surgery, there are some possible complications and risks. Your healthcare provider is the best person to tell you about the possible risks and complications. They're the best source of information because they can consider your medical history, circumstances and more.
The possible complications of surgery include:
Some complications can happen because of the leads and pulse generator. These include:
DBS uses electrical current to stimulate areas of your brain. That current almost always needs adjusting and fine-tuning before it has the best possible effects. That means the following symptoms are common while your healthcare provider is working on programming the pulse generator:
Your healthcare provider is the best person to tell you what to expect regarding your recovery time and when you will notice changes in your symptoms and how you feel. They can tell you the likely recovery time you'll need, which can vary depending on other factors like your overall health, other conditions you have and your personal circumstances.
Most people will need to stay in the hospital for one day after surgery to implant the DBS leads in their brain. Surgery to implant the pulse generator is usually a procedure where you go home the same day.
Overall, recovery time generally takes several weeks. Your healthcare provider will likely have you do the following:
Your healthcare provider will give you instructions on how to care for the areas where you had surgery. In general, you should do the following (unless they tell you otherwise):
Your healthcare provider will schedule visits to see you after your procedures. Programming visits occur with your neurologist, and you’ll need to make appointments to see them. The goal of those visits is to find the settings that work best and don't cause side effects that disrupt your life.
Regular visits with your healthcare provider are also common to monitor your condition, symptoms and to adjust medications or other treatments as needed. The schedule for these visits is something that your provider will discuss with you.
Because DBS involves surgery — especially the procedure on your brain — there are some warning signs you shouldn't ignore. You should call your healthcare provider immediately or go to the hospital outside of business hours if you have the following symptoms:
In general, deep brain stimulation is usually successful. The success rate depends on the condition involved. For conditions like epilepsy and Parkinson's disease, DBS is very effective. More research is necessary for conditions where DBS is experimental before experts know if DBS is likely to help.
In general, electronic devices and appliances shouldn’t cause any problems with the pulse generator. If they do, the most likely effect is that your pulse generator will switch off. This might not have an immediate effect, but sometimes you’ll notice that your symptoms get worse, or you'll notice an unpleasant feeling or sensation.
In general, you should keep in mind the following:
No, DBS doesn't cure conditions that it treats. It does treat the symptoms, but virtually all of the conditions that it treats are lifelong and not curable.
Depending on the disease, it may be possible to reduce medications. However, DBS is most helpful when used along with medications and other treatments. That's because using it and other treatments at the same time means it may be possible to lower medication doses, have fewer side effects and still get the same benefits.
Many insurances will cover DBS, especially if it has official approval to treat that condition. (In the United States, the U.S. Food and Drug Administration is the agency responsible for approving these uses.) It’s important that you contact your insurance company to learn if they cover DBS procedures in any way.
Deep brain stimulation (DBS) is a treatment option that can help with a wide range of conditions that affect your brain function and mental health. It’s almost always an option after other treatments and methods are unsuccessful. It’s most common for conditions like Parkinson’s disease and epilepsy, but researchers are also exploring the possibility of using it to treat many other conditions. While it does involve two to three surgeries, it’s also very effective at helping reduce symptoms and treat conditions that severely affect your quality of life.
If you have a movement or neurologic condition, deep brain stimulation might help. Cleveland Clinic’s experts can see if this treatment is right for you.

Last reviewed on 05/23/2022.
Learn more about the Health Library and our editorial process.