Ménière’s disease is a chronic inner ear disorder that leads to recurrent episodes of vertigo, hearing loss and tinnitus. Episodes may last for a few minutes up to an entire day. Symptoms worsen over time and may cause permanent hearing loss and ongoing balance issues. Treatments like medications and therapy can help manage this condition.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
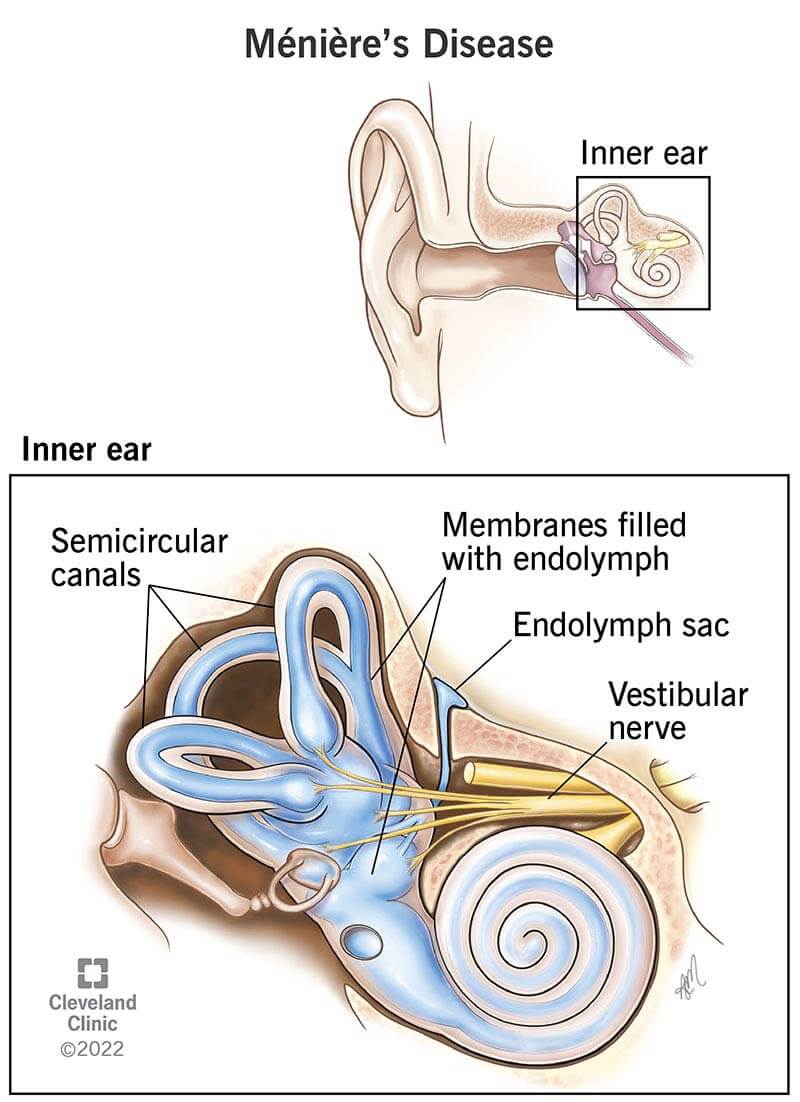
Image content: This image is available to view online.
View image online (https://my.clevelandclinic.org/-/scassets/images/org/health/articles/15167-menieres-disease)
Ménière’s disease (idiopathic endolymphatic hydrops) is a rare inner ear disorder that affects your sense of balance and hearing. It can make you feel as if the world is spinning around you when you’re standing still (vertigo). It can cause ringing in your ears (tinnitus) and difficulty hearing. Symptoms start without warning, then go away — only to come back time and time again.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Left untreated, Ménière’s disease symptoms worsen over time. They may cause permanent hearing loss and ongoing balance issues. But working with a healthcare provider to find the right treatments can help manage the condition.
Symptoms start in episodes that may last as little as 20 minutes, up to 24 hours. You may have frequent episodes back-to-back. Or, you may have long periods with no symptoms between episodes.
The main symptoms of a Ménière’s disease episode are:
Other symptoms include nausea and vomiting.
Experts don’t know what causes Ménière’s disease. But many believe that a buildup of inner ear fluid called endolymph plays a role. Too much endolymph can disrupt hearing and balance signals going to your brain.
Advertisement
Conditions that may cause too much endolymph to build up include:
More research is needed to understand the relationships between these conditions and Ménière’s disease.
Risk factors include:
Severe vertigo attacks related to Ménière’s disease can lead to serious falls. They can make everyday activities (like climbing a ladder or driving a car) too risky to attempt. Over time (usually after eight to 10 years), the condition can lead to permanent hearing loss.
Ménière’s disease can take a toll on your mental health, too. Worrying about the long-term impacts can lead to anxiety and depression.
Talk with your healthcare provider about both the physical and mental impacts of this condition, so they can help.
Otolaryngologists (ENTs) diagnose Ménière’s disease. During your visit, your provider will perform a physical exam and neurological exam. They’ll ask about your symptoms. They may do tests to rule out conditions that cause symptoms like Ménière’s disease. Tests include:
There isn’t a cure for Ménière’s disease, but treatments can reduce how severe and long-lasting your attacks are. Your healthcare provider will recommend conservative treatments first. If these treatments don’t help, you may need surgery.
Changing your routine can reduce Ménière’s disease symptoms. You can:
Healthcare providers recommend no more than 1,500 milligrams of salt daily. This is about three-quarters of a teaspoon. They may also tell you to avoid foods containing monosodium glutamate, or MSG.
Medicines for Ménière’s disease include pills you take by mouth, like:
Advertisement
If these medicines don’t help, your provider may recommend medicines you get in the form of a shot (injection). They include:
Therapies and devices that treat Ménière’s disease include:
Advertisement
Surgeries for severe cases of Ménière’s disease redirect or relieve the pressure from your inner ear fluid. Procedures include:
Ménière’s disease may go away for months or years, but it always comes back. Although there’s no cure, healthcare providers have treatments that reduce vertigo symptoms.
Follow your healthcare provider’s advice about lifestyle changes that can reduce symptoms. And always be sure to have your medications with you. Taking them when an episode starts can help you feel better sooner.
Advertisement
Having Ménière’s disease may feel like you’re being stalked by a disease that jumps out at you when you least expect it. Symptoms — like vertigo attacks and hearing problems — happen without warning. You may feel like you can’t live a normal life because you don’t know when you’ll have another attack.
Fortunately, healthcare providers have treatments that reduce Ménière’s disease symptoms. They also understand how this condition may affect your mental health. If you have this condition, ask your provider for resources that can help.

Sign up for our Health Essentials emails for expert guidance on nutrition, fitness, sleep, skin care and more.
Learn more about the Health Library and our editorial process.
Cleveland Clinic’s health articles are based on evidence-backed information and review by medical professionals to ensure accuracy, reliability and up-to-date clinical standards.
Cleveland Clinic’s health articles are based on evidence-backed information and review by medical professionals to ensure accuracy, reliability and up-to-date clinical standards.
Vertigo, dizziness and balance disorders can make you feel unsteady on your feet. Cleveland Clinic’s experts can craft a treatment plan that works for you.
