Cleveland Clinic Cancer Center (Taussig) Outcomes
Leukemia & Myeloid Disorders
Thrombocytopenic Myelofibrosis (MF) Patients Previously Treated With a JAK Inhibitor in a Phase 3 Randomized Study of Momelotinib (MMB) Versus Danazol (DAN) [MOMENTUM]
Momelotinib, an oral JAK1/2 and ACVR1/ALK2 inhibitor, showed clinical activity on MF symptoms, RBC transfusion requirements (anemia), and spleen volume in the SIMPLIFY trials, including in MF patients (pts) with thrombocytopenia. MOMENTUM is a pivotal phase 3 study of symptomatic and anemic MF pts previously treated with a JAK inhibitor (JAKi) testing MMB vs DAN. This analysis evaluated MOMENTUM pts with baseline (BL) platelet counts (PLT) ≤150 x 10⁹/L.
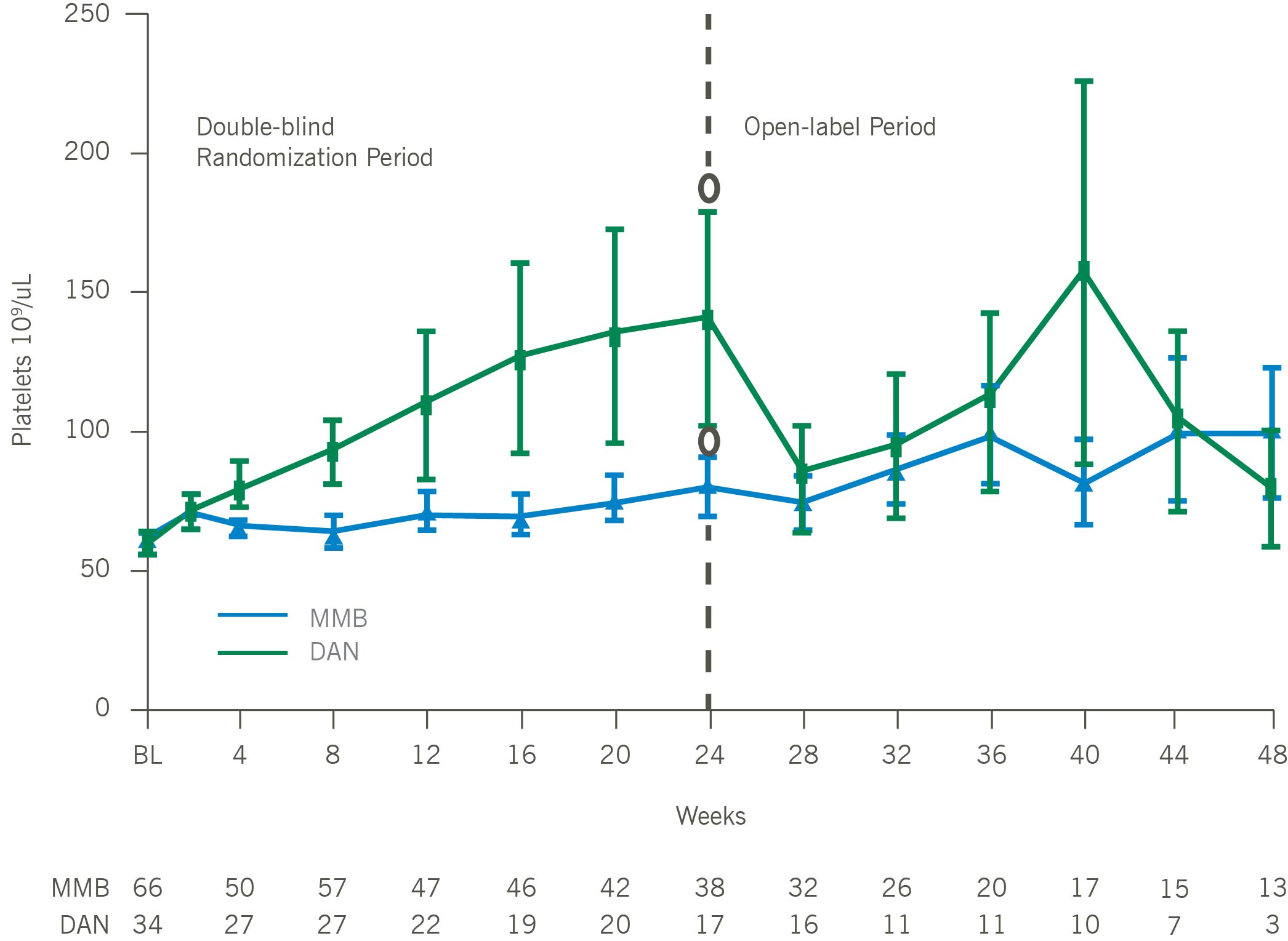
Mean Platelets Over Time for Patients With Baseline Platelets < 100 x 10⁹/uL
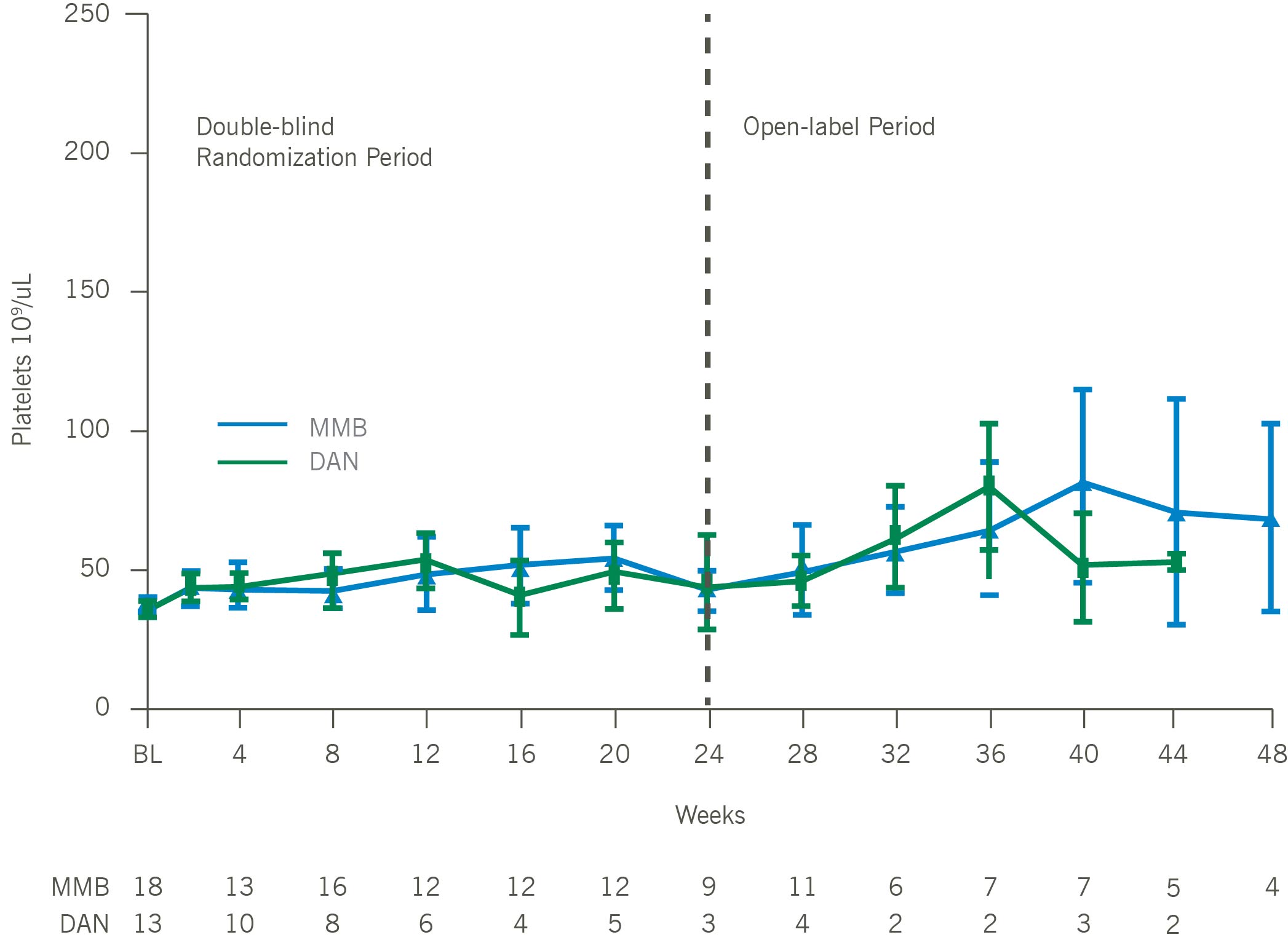
Mean Platelets Over Time for Patients With Baseline Platelets < 50 x 10⁹/uL
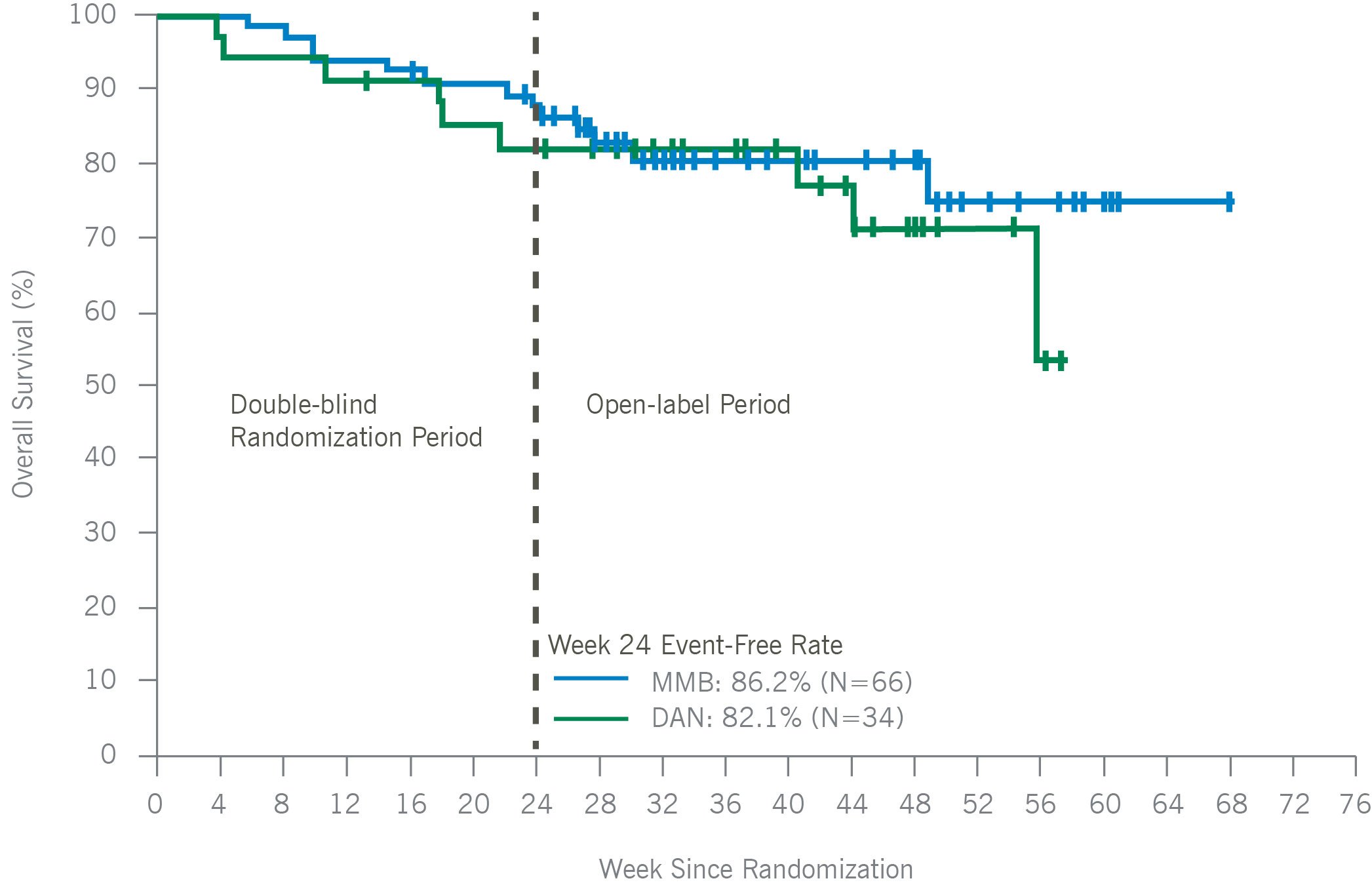
Overall Survival in Patient With Baseline Platelets < 100 x 10⁹/uL
Overall Survival in Patient With Baseline Platelets < 50 x 10⁹/uL
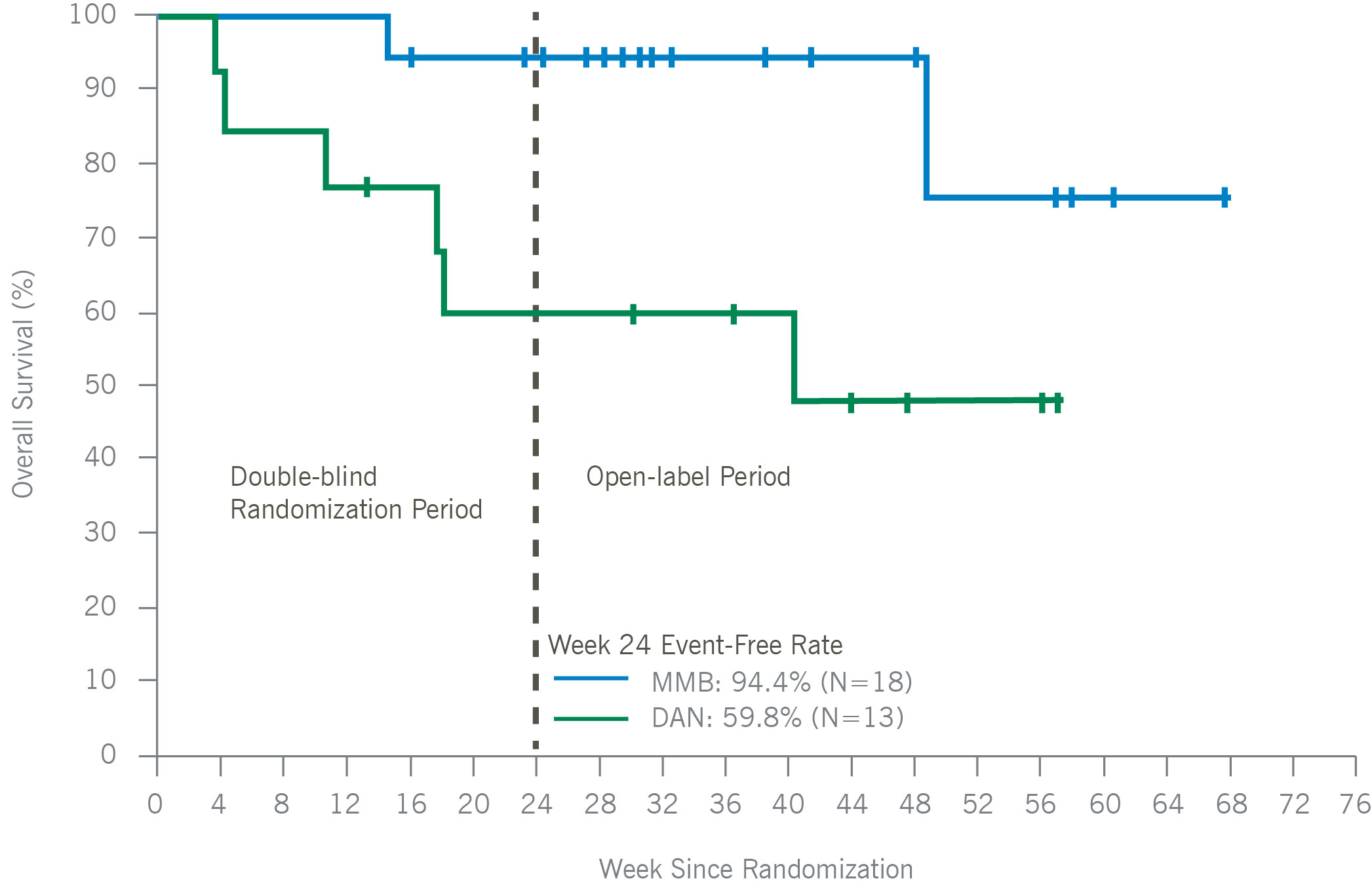
In thrombocytopenic, symptomatic, and anemic patients with MF, including those with platelets as low as 25×109/L, momelotinib was administered safely and demonstrated improvements in symptom responses, transfusion independence rates, and spleen responses as compared with danazol. Consistent with the overall intent-to-treat MOMENTUM population, platelet levels remained stable over time, and a trend toward improved overall survival versus danazol was maintained, in thrombocytopenic MF patients treated with momelotinib. Momelotinib, which is the first and only JAK1 and JAK2 inhibitor that decreases hepcidin through ACVR1 inhibition, may address a critical unmet need particularly in MF patients with symptomatic anemia and thrombocytopenia.
