
“I hate that mask!” That’s a typical, frustrated reaction for 40 percent of obstructive sleep apnea (OSA) patients who are uncomfortable with the gold standard treatment for the sleep disorder — continuous positive airway pressure (CPAP). CPAP requires use of a nose- or mouth-covering mask that delivers the patient constant, steady air pressure to limit breathing issues while asleep.
But many – including Sharon Ward — struggle with how the mask fits, can’t tolerate continuous air, or are claustrophobic wearing the mask. “It didn’t fit right on my small face, and I had anxiety using it,” recalled Sharon, 69, of Canal Winchester, Ohio. “After two years of going back and forth to the doctor, I just didn’t use it anymore.”
With a history of heart disease, Sharon, by abandoning the therapy, placed herself at risk for hypertension, stroke and cardiac problems often associated with sleep disorders. Now, however, she is successfully using a viable alternative: the FDA-approved upper airway stimulation (UAS) device.
A research study published in the European Respiratory Journal, co-authored by Cleveland Clinic sleep specialist Reena Mehra, MD found UAS demonstrated substantial reduction in the severity of OSA and high levels of patient-reported satisfaction with the device.
“Left untreated, OSA can negatively impact the quality of life or cause significant medical problems,” noted Dr. Mehra, Director of Sleep Disorders Research in the Sleep Center at Cleveland Clinic’s Neurological Institute. “The study results support that UAS is an effective alternative therapy for treatment of sleep apnea in specific patients.”
The new device, which is surgically implanted into the patient’s body while he or she is under general anesthesia, uses UAS to open key airway muscles throughout the night. Controlled by a remote or wearable patch, its system includes a breathing sensor and a stimulation lead powered by a small battery. During sleep, it senses breathing patterns and delivers mild stimulation to the tongue and throat to keep the airway open.
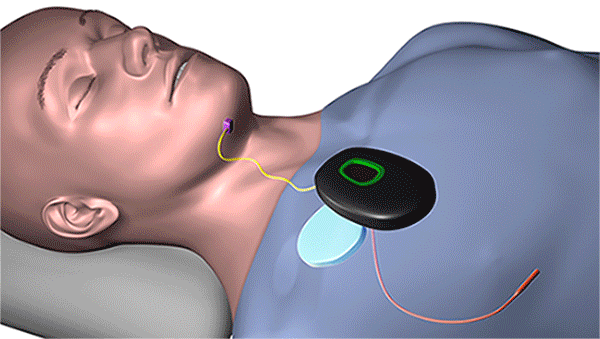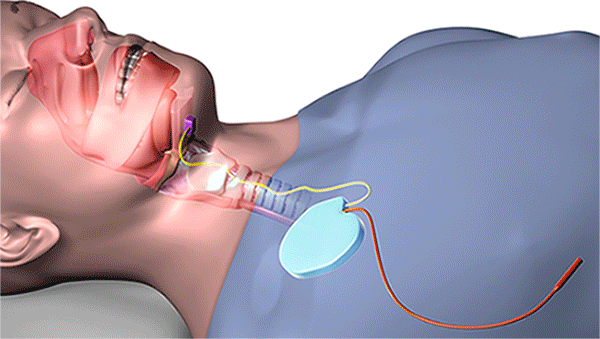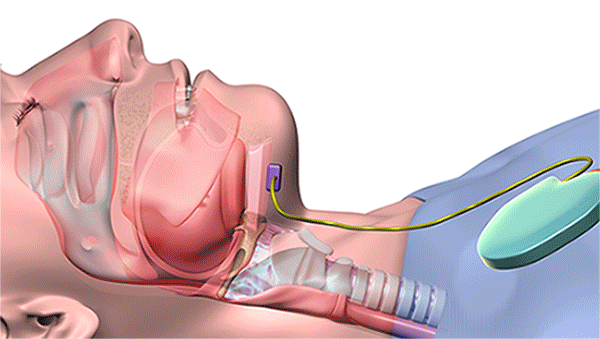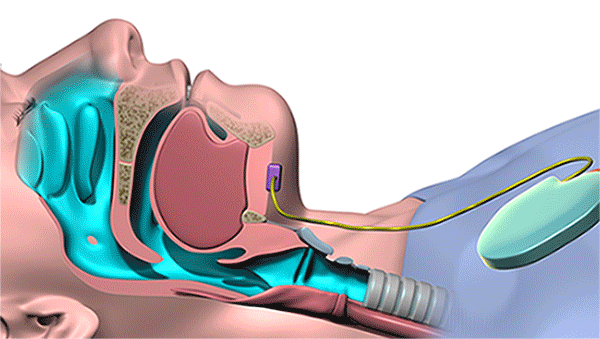
Using a remote, the implantable device synchronizes the tongue and palate movements to open key airway muscles throughout the night. (Courtesy: Cleveland Clinic)
While not all OSA patients are eligible for the device, which requires a diagnosis of moderate or severe OSA among other criteria, the study revealed the effectiveness of UAS is higher in women and older patients. The technology was named to Cleveland Clinic’s Top 10 Medical Innovations of 2018.
Among the first of more than 3,000 patients to have had the UAS device surgically implanted. Sharon had the device implanted by surgical sleep medicine specialist, Alan Kominsky, MD. She noticed an immediate improvement after she began using the remote-controlled device.
“I noticed a change with my sleeping right away. I slept all night and didn’t snore. No more nightmares or gasping for breath,” she explained. “When I wake up, I don’t feel sleepy or groggy. The quality of my sleep — and life — is so much better.”
Related Institutes: Neurological Institute

