Head & Neck Institute Outcomes
Laryngology
The Evolution and Outcomes of the Maddern Procedure for the Treatment of Subglottic Stenosis
The Maddern Procedure, a novel, minimally invasive technique to treat subglottic stenosis, has been gaining acceptance in academic centers. The study below describes the technique, as well as shares insights on its evolution over the first 28 patient cases performed at the Cleveland Clinic.
A prospective case-series cataloged descriptive technique modifications for the procedure and was developed after surgeons accumulated and followed a patient cohort for 6 years with a minimum of 2 years of follow-up care (11/2015-11/2021). Main outcomes examined included changes to surgical indications, complications, and post-operative results using validated measures of voice and breathing.
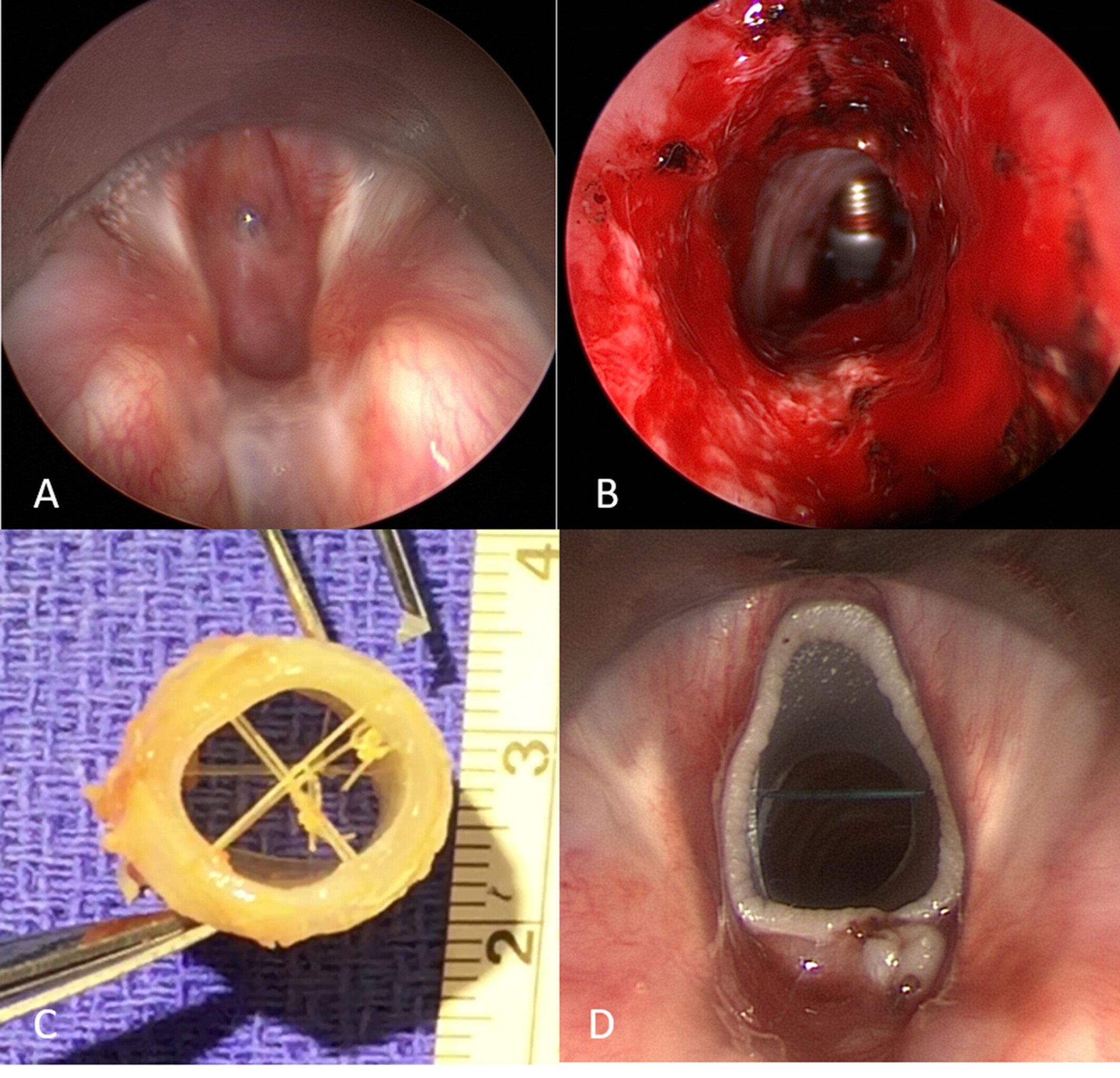
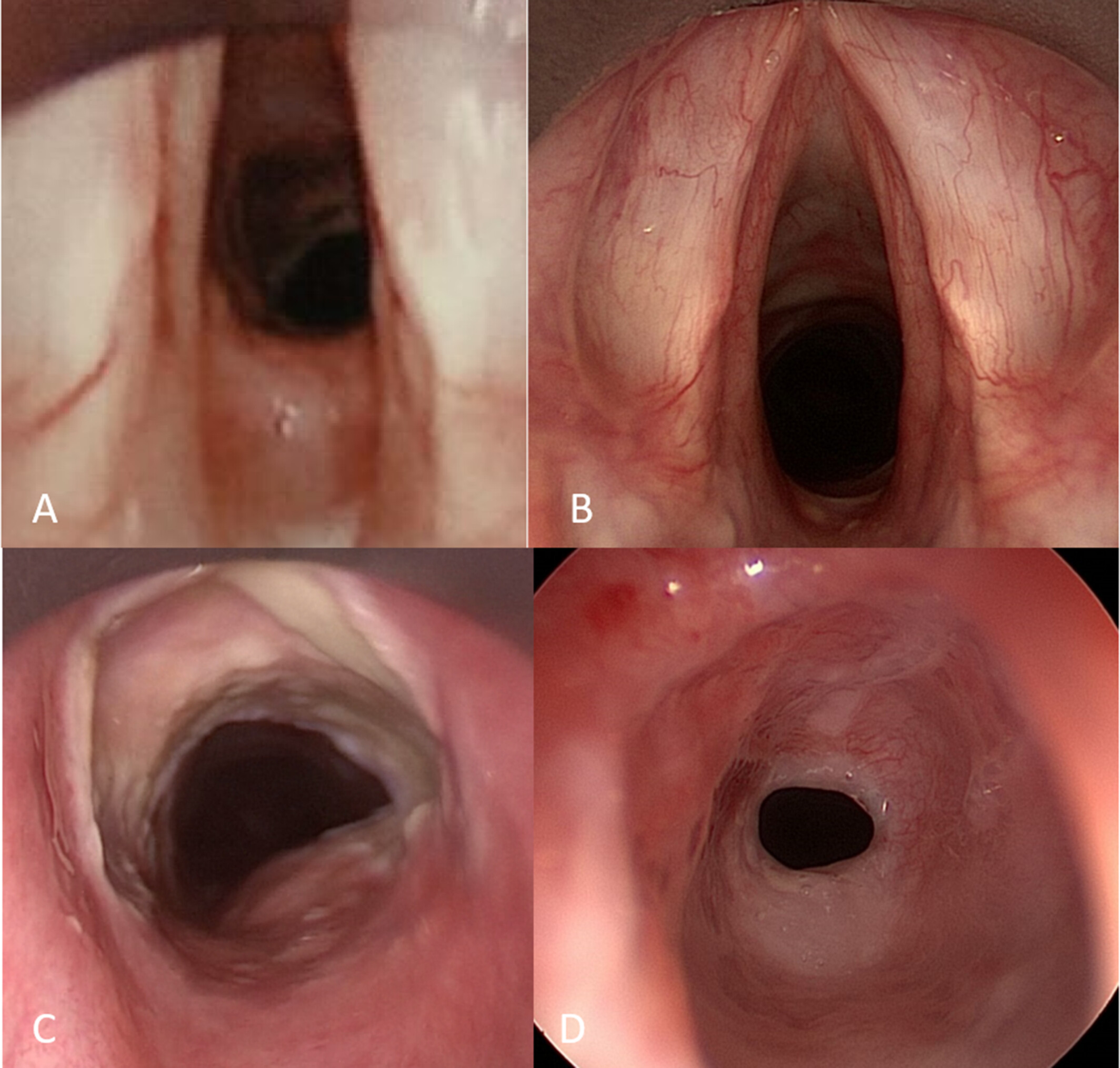
Complete resection of subglottic scaring was performed, at first transcervically (2 patients), then transorally (26 patients). Successful performance of the procedure occurred in all patients without complications, with either successful decannulation of previously existing tracheotomies or removal of perioperative tracheotomies. Buccal grafts in 8 patients replaced skin grafts as the graft of choice. Although high subglottic disease was first thought to be a contraindication, superior results became evident in cases of high stenosis rather than disease that included the upper trachea, with 4 out of 26 patients requiring subsequent tracheal resection or tracheal dilation. Of the 22 remaining patients, 19 had successful arresting of restenosis, with 2/22 undergoing subsequent cricotracheal resection, and 1patient requiring subglottic dilation. Overall, 19/26 (73 percent) patients undergoing the Maddern procedure had objectively favorable outcomes, with 24/26 (92 percent) reporting that if they had to do it all over, they would make the same decision and select to undergo the procedure again.
Although technically challenging, full-thickness mucosal resection and relining of the subglottis is a developing technique that is a safe procedure with favorable patient outcomes that address the recurrent nature of the disease.
Outcome Measures
Patient # | Months f/u | Hosp LOS | Sub Rx | Sub surg interv months | VHI-10 | PCS | MCS | Improve 1-10 | Again? Y/N |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 76 | 3 | SD | 1 | 3 | 60.0 | 31.5 | 10 | Y |
| 2 | 75 | 2 | 0 | 62.7 | 22.7 | 10 | Y | ||
| 3 | 71 | 1 | 0 | 61.5 | 30.6 | 10 | Y | ||
| 4 | 69 | 1 | 2 | 56.4 | 60.0 | 10 | Y | ||
| 5 | 68 | 2 | 0 | 54.1 | 40.4 | 10 | Y | ||
| 6 | 67 | 6 | 6 | 56.7 | 62. | 10 | Y | ||
| 7 | 60 | 1 | TR | 27 | 0 | 56.7 | 62.4 | 10 | Y |
| 8 | 58 | 1 | 2 | 57.0 | 46.8 | 10 | 7 | ||
| 9 | 58 | 1 | 0 | 48.9 | 61.1 | 9 | Y | ||
| 10 | 57 | 3 | 2 | 53.5 | 63.4 | 10 | Y | ||
| 11 | 57 | 1 | 2 | 56.7 | 62.4 | 10 | Y | ||
| 12 | 55 | 1 | 32 | 33.0 | 19.3 | 8 | Y | ||
| 13 | 55 | 1 | TR | 12 | 0 | 53.9 | 56.2 | 9 | Y |
| 14 | 55 | 1 | CTR | 26 | 37 | 49.1 | 47.0 | 2 | N |
| 15 | 55 | 2 | 0 | 32.9 | 63.8 | 10 | Y | ||
| 16 | 55 | 1 | 16 | 32.9 | 45.9 | 9 | Y | ||
| 17 | 54 | 1 | 8 | 50.9 | 42.0 | 10 | Y | ||
| 18 | 53 | 1 | 15 | 56.1 | 57.2 | 7 | Y | ||
| 19 | 53 | 1 | TD | 53 | 0 | 46.2 | 42.2 | 4 | Y |
| 20 | 52 | 3 | 0 | 42.3 | 37.4 | 8 | Y | ||
| 21 | 51 | 2 | TD | 9 | 0 | 57.8 | 57.1 | 8 | Y |
| 22 | 50 | 3 | 5 | 52.5 | 59.1 | 10 | Y | ||
| 23 | 46 | 1 | 0 | 52.8 | 57.1 | 10 | Y | ||
| 24 | 43 | 4 | CTR | 6 | 12 | 57.5 | 54.2 | 5 | N |
| 25 | 40 | 2 | 0 | 55.1 | 62.5 | 10 | Y | ||
| 26 | 39 | 5 | SD | 2 | 1 | 52.2 | 51.0 | 2 | Y |
| 27 | 31 | 2 | 9 | 57.5 | 54.2 | 9 | Y | ||
| 28 | 27 | 3 | 4 | 52.2 | 56.8 | 9 | Y |
Number of months of follow-up, length of hospital stay (days), subsequent procedures including tracheal resection (TR), cricotracheal resection (CTR), tracheal dilation (TD), or subglottic dilation (SD), number of months interval from Maddern to subsequent surgery, VHI-10 score at followup, SF12v2 Physical Component Score, SF12v2 Mental Component Score. The patients' subjective score of Maddern procedure improving breathing quality (1 minimally–10 maximally), and yes/no answer to “would you have gone through the Maddern procedure knowing what you know now?”
