Mary Beth Zolik-Smith, her husband, Terry, and their three children have a lot to celebrate. Mary Beth is cancer-free from the non-Hodgkin lymphoma she has valiantly fought since 2012.
“We have great joy in our hearts,” Mary Beth exclaims. “There is so much to celebrate. Sometimes, I can’t believe how my life has changed.”
Mary Beth’s cancer is in complete remission following participation in a multi-center clinical trial of a cell-based gene therapy to treat adults with certain types of large B-cell lymphoma who have not responded to or who have relapsed after at least two other kinds of treatment.
The breakthrough chimeric antigen receptor CAR T-cell therapy has since become the second gene therapy approved by the U.S. Food and Drug Association (FDA) and the first approved for certain types of non-Hodgkin lymphoma.
According to Mary Beth’s medical oncologist, Dr. Brian Hill of Cleveland Clinic Cancer Center, the customized treatment is a cancer medicine made from the patient’s own white blood cells to stimulate the immune system to help fight the lymphoma.
“Historically, patients like Mary Beth with this form of cancer had very few options for curative therapy if a stem cell transplant didn’t work,” explains Dr. Hill. “She was an ideal candidate for the clinical trial, and had a great response – all detectable areas of cancer have disappeared.”
When diagnosed in 2012, Mary Beth underwent successful chemotherapy treatment. But her remission only lasted a few years, and her large B-cell lymphoma returned. When a stem cell transplant – a way of delivering high-dose chemotherapy – failed to work, Mary Beth urged her Toledo physician to explore clinical trials she had read about in researching her form of cancer.
And that effort, combined with her own diligence in finding another treatment option, led her to the Cleveland Clinic and Dr. Hill. She recalls the process of applying for and being evaluated for the clinical trial as nerve wracking, like awaiting the results of a final exam in college: “You have to be sick, but not too sick. You can’t have an infection. A lot of things can derail you.”
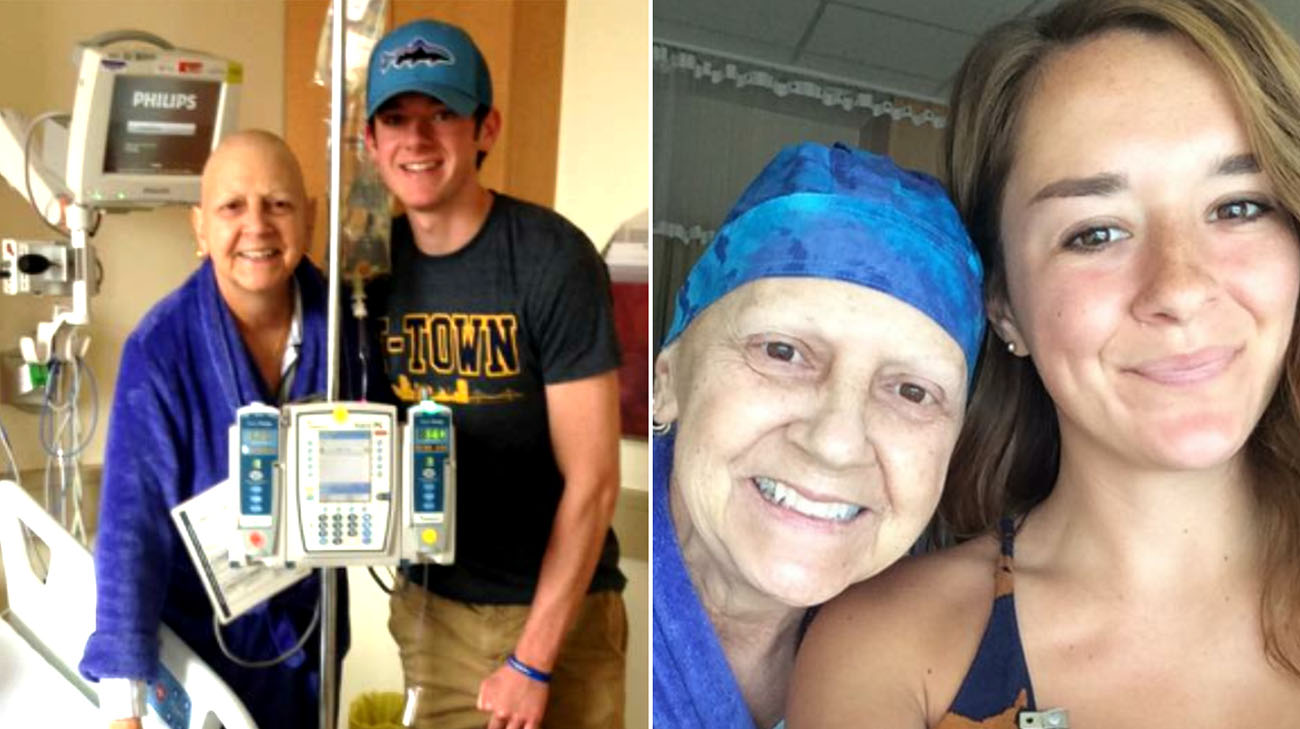
Mary Beth with her son and daughter while in the hospital for treatment. (Courtesy: Mary Beth Zolik)
Once accepted for the trial at the Cleveland Clinic, Mary Beth first underwent a procedure to collect white blood cells from her body. They were then shipped to California, where they were genetically modified in a laboratory with the receptor that would enable her body to recognize and fight the cancer.
Mary Beth likens CAR T-cell therapy to a video game: “They take your T-cells out of your body, fire them up, and reinsert the T-cells into your body. And they become your warriors. They go right after the cancer in your system and just gobble it up.”
While this process was taking place, Mary Beth underwent a few days of chemotherapy to condition her immune system to better accept the modified cells, which were ultimately transplanted back into her body. While she did eventually experience a temporary side effect – a neurologic toxicity that left her in a confused state for about 36 hours – the treatment worked perfectly.
Mary Beth was among the 51 percent of patients treated in the clinical trial to experience complete remission – something she knows wouldn’t have occurred if she hadn’t sought out and found a clinical trial.
“You have to think of yourself not as a patient but as a pioneer,” adds Mary Beth. “A year ago, I didn’t know if I’d still be alive today. It shows the importance of being an advocate for yourself.”
Related Institutes: Cleveland Clinic Cancer Center

