Definition & Nomenclature
Jamile Wakim-Fleming MD, FACG, FAASLD
Nicholas S. Hanlon, MD
William D. Carey, MD
Definition and Nomenclature
Nonalcoholic fatty liver disease (NAFLD) encompasses a spectrum of conditions associated with > 5% lipid deposition in the liver. The spectrum ranges from simple steatosis characterized by the innocuous presence of fat droplets in hepatocytes, to the more advanced form of nonalcoholic steatohepatitis (NASH), and the more progressive and severe form of cirrhosis.
Healthy liver ↔ Steatosis ↔ NASH ↔ Fibrosis ↔ Cirrhosis
Histologically, NASH is characterized by steatosis, inflammation, ballooning, and injury to hepatocytes. NASH heralds the progression to cirrhosis, but it can be reversed with appropriate interventions. When cirrhosis supervenes, fat may disappear on histologic studies. This condition is reported as burned out fatty liver or cryptogenic cirrhosis.
Advances in understanding the pathophysiologic mechanisms underlying the development of fatty liver disease led to the knowledge that NASH is associated with insulin resistance and is currently considered by many to be the hepatic component of the metabolic syndrome.
The presence of fat in hepatocytes is seen in many conditions (Table 1) most especially excessive alcohol intake. These conditions should be excluded and corrected before the diagnosis of NAFLD is made. Further, the pathological changes seen on liver biopsy specimens in NAFLD may not be clearly differentiated from those seen in alcoholic liver injury, and the diagnosis of NAFLD is only considered in the absence of excessive alcohol intake.
Table 1
| Medications associated with fatty liver | Disorders associated with fatty liver | Nutritional factors associated with fatty liver |
| Amiodarone | A-betalipoproteinemia | Rapid weight loss |
| Corticosteroids | Lipodystrophies | Total parenteral nutrition |
| Methotrexate | Hypopituitarism | Starvation |
| Tamoxifen | Wilson disease | Protein-calorie malnutrition |
| Vitamin A | Hep C | |
| HIV antiretroviral agents | Alcohol |
The CDC defines excessive alcohol use as heavy drinking of 15 standard drinks or more per week for men, and 8 standard drinks or more per week for women. A standard drink is 14.0 grams (0.6 ounces) of pure alcohol. This is found in 12 ounces of beer (5% alcohol content), 8 ounces of malt liquor (7% alcohol content), 5 ounces of wine (12% alcohol content), 1.5 ounces or a “shot” of 80-proof (40% alcohol content) distilled spirits or liquor (e.g., gin, rum, vodka, whiskey).
Epidemiology & Natural History
Metabolic syndrome including NAFLD is becoming one of the leading causes of cirrhosis, liver transplantation and kidney transplantation due to renovascular disease. NAFLD is the fastest growing cause of hepatocellular carcinoma (HCC) among transplant candidates on the waiting list. A 75% increase in incident cases of HCC is reported between 1990 and 2015 owing to a combination of ageing population and increase in metabolic syndrome.
NAFLD is more common in men than women, in Hispanics than Whites and African Americans, and it increases in age but decreases in the elderly. The presence of hepatic fibrosis is the strongest predictor of long term outcome. Advanced fibrosis is shown in a prospective study to be associated with liver related complications and death.
Children and adults with fatty liver disease have similar clinical characteristics and NAFLD children are very likely to become NAFLD adults, but they differ on histopathologic findings on liver biopsy.
Several factors influence the development of fatty liver disease. These include genetic factors, sedentary lifestyle and hyper-caloric diets. Therefore, NAFLD can be seen in obese and in non-obese lean individuals who have features of the metabolic syndrome. Metabolic syndrome (Table 2) is defined by a combination of abnormal Body Mass Index (BMI), enlarged abdominal waist, dyslipidemia (high triglycerides and low HDL), type 2 diabetes/ insulin resistance and hypertension.
Table 2: Metabolic syndrome is diagnosed by the presence of 2 or more of these parameters as defined by WHO.
| Impaired glucose tolerance | Fasting blood glucose level ≥ 110 mg/dL or taking glucose-lowering medication |
| Hypertension | ≥ 130/80 mm Hg |
| Hypertriglyceridemia | > 150 mg/dL |
| Low high-density lipoprotein level |
< 40 mg/dL for men < 50 mg/dL for women |
| Abdominal (central) obesity |
Waist circumference > 102 cm (40”) for men Waist circumference > 88 cm (35”) for women |
The prevalence of NAFLD is rising in parallel with the rise in obesity rate. Obesity defined as a BMI ≥30 kg/m2 is present in more than 30% of the general population in the US and worldwide (with some geographic variations) and it affects children as well as adults. Obesity rate is expected to reach 48.9% by 2030. Similarly, NAFLD affects as much as 30-40% of the general population in the US, 75% in people with obesity and > 90% in people with morbid obesity (BMI > 40). NAFLD prevalence is increasing with age and is reported in 34% of the population over 70 years old. It is twice as common in individuals with type 2 diabetes (T2D) compared to the general population, and this combination is associated with advanced fibrosis and increased cardiovascular risks and mortality.
In lean individuals with a BMI < 25, NAFLD is reported between 5% - 45% with an average of 20%. Lean individuals may have central obesity or other metabolic risks.
While the majority of patients with NAFLD have simple steatosis, 4% develop NASH over at least 10 years. An average of 3% - 6% of patients with NAFLD have NASH, and 22% of patients with NASH progress to cirrhosis over at least a 10-year period. It is also reported that up to 5% of patients with NASH may spontaneously regress over time. In patients with T2D, 37% have NASH and 17% - have advanced fibrosis on liver biopsy. In patients with incidentally discovered steatosis, 11% might be at high risk for advanced hepatic fibrosis.
Coronary artery disease and malignancy followed by liver-related mortality are the most common causes of death in people with NASH.
The estimated annual incidence of HCC in NASH cirrhosis ranges between 0.5% - 2.6%, and between 0.1 - 1.3 per 1000 person-year in non-cirrhotic individuals. Although these numbers may appear small, the absolute numbers ae important considering the prevalence of the disease.
NAFLD recurs in 80 - 100% of patients post liver transplant and occurs de novo in about 50%. Rate of progression to cirrhosis is reported to be higher in post liver transplant (LT) than before LT in NASH transplanted patients. The presence of NASH is independently associated with an increased risk of death from cardiovascular diseases (CVD) and most CV events (39%) occur in the first year after LT.
Pathophysiology
Several insults to the liver drive the progression from simple steatosis to cirrhosis in NAFLD. But it is unclear which metabolic alterations trigger NASH-promoting pathways and why the majority of steatotic livers do not progress to NASH.
One widely accepted pathway is insulin resistance. Insulin resistance represents a decreased glucose uptake by the liver, increased gluconeogenesis, mitochondrial injury and de-novo lipogenesis. This in turn increases circulatory free fatty acids (FFAs) and FFA uptake by the hepatocyte. As a result, an environment of lipotoxicity occurs associated with a state of imbalance between synthesis and degradation of lipids in the liver.
In genetically predisposed individuals with NASH, it is postulated that lipotoxicity and lipid dysregulation in concert with microbiome signaling contribute to metabolic dysregulation and the inflammatory state in NAFLD. Activation of macrophages and hepatic stellate cells along with the release of a cascade of inflammatory mediators such as (TNF)-alpha, Notch, Hh, JNK, NFKB, hepatokine, IL6 and natural killer cells, act not only on the liver to perpetuate liver damage and fibrogenesis, but also systemically on extrahepatic tissue to contribute to atherosclerosis and the development of CVD, kidney injury, cancers and neurocognitive dysfunction.
Success of genome wide association technology studies on polymorphisms of metabolic diseases has enabled the identification of key genes involved in the development of fatty liver disease. The most commonly described NAFLD risk genes are the PNPLA3, the TM6SF2 and the MBOAT7 genes and their variants. The PNPLA3 bears a higher risk for liver disease but a lower risk for CVD probably due to associated lower lipid levels.
It is becoming clear that circadian rhythms, influenced by ambient light and regulated by supra-chiasmic nucleus, modulate sleep/wake cycle, temperature, feeding and fasting homeostasis. Two types of circadian rhythms are described: peripheral (liver) and central (hypothalamus). They play a role in physiology, metabolism of drugs, and fatty liver disease via circadian clock proteins. These proteins help regulate transcription of genes that influence cholesterol, bile, glucose, and lipid metabolism. Normally circadian rhythms function in balance, but in NAFLD, a dys-synchrony develops between central and peripheral circadian rhythms. Correction of this dys-synchrony by restricting feedings to a defined daily interval along with behavioral interventions is reported to restore synchrony and prevent, even treat the metabolic syndrome.
Gut microbiota dysbiosis is reported in NAFLD, T2D, and obesity. There is decreased richness in gut microbial genes that is associated with reduced bacteria-producing short chain fatty acids, and increased bacterial lipopolysaccharides. This in combination with increased intestinal permeability in NAFLD contribute to a pro-inflammatory status and worsened adiposity and insulin resistance. Gut microbiota modifications through the ingestion of polyphenols, and a Mediterranean-like diet low in saturated fats has shown to reverse or improve NAFLD.
Diagnosis
Clinical evaluation and diagnosis
NAFLD can be a silent disease that progress to cirrhosis without symptoms. Therefore, the diagnosis of fatty liver disease should be pursued when a clinical suspicion of fatty liver arises, in the presence of unexplained abnormal liver panel, when fatty liver is seen on imaging studies or when a patient has insulin resistance and at least 2 features of the metabolic syndrome. NAFLD is often discovered when laboratory examination incidentally reveals elevated aminotransferase levels, or steatosis on liver US. However, liver enzymes are not sensitive nor specific for the diagnosis of NAFLD or NASH.
Signs and symptoms
NAFLD produces no symptoms in most. When symptomatic, patients report malaise, fatigue or vague abdominal discomfort. Occasionally pruritus is reported. Hepatomegaly may be found on clinical examination and in cirrhotic patients, ascites, spider angiomas, splenomegaly, palmar erythema, and asterixis can be present.
Associated comorbidities can be present in varying degrees and include obesity in 47% - 90%, diabetes mellitus in 28% - 55%, cardiovascular disease, dysplipidemia, obstructive sleep apnea, polycystic ovarian syndrome, osteoporosis, hypopituitarism and hypertension. Cardiovascular disease reported as arrhythmias, coronary artery disease, cardiomyopathy and hypertension significantly increases morbidity and mortality in individuals with NAFLD.
Laboratory Studies
Liver enzymes can be normal, mildly or moderately elevated. Albumin, bilirubin, and platelet levels are usually normal unless the disease has progressed to cirrhosis. It is not uncommon to find mild abnormalities in autoimmune antibodies and iron studies associated with NAFLD. The mechanisms are not totally clear.
Conditions associated with fatty liver should be excluded and laboratory work up for abnormal liver enzymes undertaken. Serum lipid panel and fasting glucose or hemoglobin A1c should be checked and treated.
Imaging
The normal liver contains less than 5% fat.
Liver ultrasound is useful as a screening test when a suspicion of fatty liver is raised. The liver appears diffusely echogenic (bright liver) and the vasculature is blurred.
Fibroscan® provides two important metrics: an estimate of hepatic fibrosis (as estimated by elastography or liver stiffness measure LSM, reported in kPa), and of fat content measured via the Controlled Attenuation Parameter (CAP score). It represents an excellent screening tool to exclude significant hepatic fibrosis.
According to Baveno VII workshop, LSM values < 10 kPa rules out compensated advanced chronic liver disease cACLD, whereas LSM values >15 kPa are highly suggestive of cACLD.
A study by Siddiqui et al, showed that LSM values < 6.5 kPa excluded advanced fibrosis with a negative predictive value of 0.91; a cut-off LSM of 12.1 kPa excluded cirrhosis with a negative predictive value of 0.99.
A reasonable inference can be made that simple steatosis, is present with Fibroscan® elastrography LSM values < 8 kPa and that LSM values > 15 kPa correlate with advanced fibrosis.
NAFLD Fibroscan® elastrography values between 8-15 kPa, do not provide a clear signal about the degree of hepatic fibrosis. In such cases, biomarkers described below are used to assign likelihood of fibrosis. In some cases liver biopsy or advanced imaging modalities may be needed.
Fibroscan® data has been used in the majority of studies of fatty liver disease. Although other means of elastrography are available, conversion of kPa data among modalities is not possible.
MRElastography and MR Spectroscopy-Proton Density Fat Fraction MRSPDFF are additional imaging studies that have been used in the diagnosis of NAFLD. They are more accurate than Fibroscan® in diagnosing advanced fibrosis and cirrhosis.
MRSPDFF is shown to be highly accurate in assessing fibrosis stage and a very low degree of steatosis approximating 6%. It can map the entire liver, but cannot distinguish steatosis from NASH. Is use is limited by high cost, making it not suitable as a screening test.
Ultrasound examination using a Two-Dimensional Shear Wave Elastography and Attenuation Imaging has the potential to identify fibrosis stage.
Bio-markers of fibrosis:
Multiple markers are useful adjuncts in assessing the degree of fibrosis in NAFLD and provide prognostic significance. Some are simple and others are complex. Some are proprietary (e.g., Fibrotest, ELF, LiverFast and BARD) and therefore costly and results are not available immediately. Proprietary test have not been shown to be superior to non-proprietary tests and are not further considered here. Simple scores are non-proprietary and can be easily calculated by just plugging the different components in the web. They are adopted by major societies and include APRI, NFS and Fib4. (Table 3)
Table 3: Biomarkers of fibrosis
| Test | Values | Web Calculator |
| APRI (AST to Plat. Ratio Index) | AST, platelets | https://www.hepatitisc.uw.edu/page/clinical-calculators/apri |
| NFS (NAFLD Fibrosis score) | Age, Blood glucose, BMI, platelet count, albumin level, and AST/ALT ratio | https://www.mdcalc.com/calc/3081/nafld-non-alcoholic-fatty-liver-disease-fibrosis-score |
| Fib 4 (Fibrosis 4) | Age, platelet count, ALT level, and AST level | https://www.hepatitisc.uw.edu/page/clinical-calculators/fib-4 |
Liver Biopsy (Figure 1)
Biopsy remains the most accurate means to identify steatohepatitis, but it is invasive and not without side effects. Liver biopsy is recommended when competing etiologies for liver disease are suspected but cannot be diagnosed or excluded by other means. Liver biopsies are evaluated based on the degree of steatosis, inflammation, ballooning of hepatocyte and fibrosis. Findings are reported as the NAFLD activity score (NAS). A NAS > 5 or the presence of ballooning identifies NASH. Liver biopsy in adults shows a centrizonal disease, whereas in children, the disease affects the periportal zone.
Liver biopsy cannot clearly differentiate between alcoholic and non-alcoholic steatohepatitis and other means of diagnosing alcoholic liver disease should be pursued.
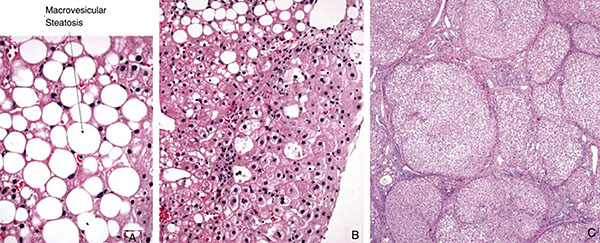
Figure 3: Spectrum of disease in NAFLD. A: Simple steatosis. B: Nonalcoholic steatohepatitis (NASH). C: Cirrhosis. (Image courtesy of Lisa M. Yerian, MD).
When a liver biopsy is not feasible or desirable, a diagnostic stepwise approach and risk assessment is recommended that includes fibrosis markers in addition to liver stiffness measurement or fibrosis by other imaging modalities.
Determining risk for advanced fibrosis:
Since liver fibrosis is the most important determinant of long term outcome and mortality, it is important to determine the degree of fibrosis and risk for progression in order to institute early interventions and guide treatment strategies to mitigate the development of cirrhosis and the morbidity and mortality associated with it. Laboratory testing, imaging studies and markers of fibrosis can be used to determine risk for progression of the disease without recourse to liver biopsy:
- Individuals who have simple steatosis or a Fib-4 < 1.3 and LSM < 8 Kpa are at low risk for advanced fibrosis or decompensation and can be managed conservatively with lifestyle modification.
- Individuals who have a Fib-4 between 1.3 - 2.67 and LSM between 8-15 Kpa, have an intermediate risk of advanced fibrosis and should be followed closely and evaluated on regular intervals with repeated Fibroscan®, platelet counts and liver panel, or advanced imaging modalities such as MRE to further define their disease progression and institute appropriate measures.
- Individuals with a Fib-4 > 2.67 and LSM > 15 Kpa, or LSM > 20 Kpa are at high risk of cirrhosis and decompensation. They should undergo aggressive therapy.
Management
Life style Modification and weight loss: The backbone in the management of individuals with NAFLD in all risk categories is putting emphasis on lifestyle modifications via avoidance of alcohol, a significant weight loss of 5-10% and regular aerobic physical activity. Physical activity can be of moderate intensity for about 150-300 min weekly or vigorous intensity for 75 -150 minutes weekly, or a mix of both. A significant weight loss requires a reduction of 500-1000 Kcal per day.
A weight loss of > 5% has shown to improve steatosis, and a weight loss> 7% can lead to resolution of NASH. Some degree of fibrosis regression can be seen with a greater weight loss of > 10%.
Studies have established that lifestyle modification and decreased liver fat and fibrosis in NAFLD patients, improve insulin sensitivity, reduce risk of diabetes and cardiovascular disease, as well as decrease the risk of progressive liver disease and cirrhosis related deaths and HCC. Due to risk of muscle mass loss, worsening sarcopenia, and nutrient deficiencies, it is advisable to seek the assistance of nutrition specialists for a personalized plan of care.
Pharmacologic therapies with FDA approved agents for Obesity may be necessary adjuncts when lifestyle modification alone fails to reduce weight appropriately.
Alcohol: There is no clear data on the effect of light or moderate alcohol consumption in NAFLD patients, and abstinence is strongly recommended when advanced fibrosis is present.
Coffee: The ingestion of 3-4 cups of coffee a day has shown to decrease gut permeability, improve fibrosis and decrease inflammation in NASH patients and is highly advisable.
Pharmacologic Interventions
Pharmacologic therapies specifically targeting factors involved in the pathogenesis of NASH and aiming to resolve steatosis and fibrosis should be considered.
Statins and PPAR agonists target fat accumulation and the metabolic features; lifestyle modification, medical, endoscopic or surgical intervention aim for weight reduction; vitamin E and lipophilic statins aim to diminish oxidative stress and inflammation. Psychosocial interventions aim to manage stress and wake-sleep cycle. Whereas immune modulators, modulators of gut microbiota and antifibrotic agents are still being investigated, a holistic approach is desirable with the goal to improve quality of life, target liver disease to prevent the development of cirrhosis and its complications, and prevent the systemic inflammation associated with the metabolic syndrome most notably cardiovascular and renal complications.
Vitamin E mechanism in NAFLD is not totally elucidated but it has antioxidant and anti-inflammatory properties. A prospective trial (PIVENS trial) that included a treatment arm of Vitamin E 800 IU daily showed that Vitamin E improves liver histology in non-diabetics with biopsy-proven NASH. A recent study showed that vitamin E can also be given to people with diabetes. In this retrospective propensity matched study where data was captured from the outpatient electronic medical records of patients with NASH and at least bridging fibrosis, Vit E 800 IU daily showed a higher adjusted transplant-free survival and lower rates of decompensation in patients with or without diabetes.
Vitamin E at 800 IU/day can be considered in NAFLD patients unless there is a strong family history of prostate cancer or in patients taking concomitant blood thinners, as they can increase risk of hemorrhagic stroke and all-cause mortality.
Lipid lowering agents: statins inhibit 3 hydroxy-3-methylglutaryl-coenzyme-A reductase, a key enzyme involved in cholesterol synthesis, bile and lipid metabolisms. They also possess anti-inflammatory properties, improve endothelial function, increase the synthesis of nitric oxide, and the number of endothelial progenitor cells, among other potential effects. Statins are indicated to treat dyslipidemia and improve cardiovascular health.
Importantly, a systematic review and meta-analysis studies of lipophilic statins (simvastatin, fluvastatin, lovastatin, atorvastatin, pitavastatin) in patients with liver disease suggest a beneficial effect on risk of hepatic decompensation, progression of liver disease, mortality and development of liver cancer without significant additional costs. A recent large retrospective study involving over 7.8 million individuals, showed that the use of statins is significantly associated with a dose dependent risk reduction of NAFLD and of fibrosis and a beneficial effect on the progression of disease.
Considering their beneficial effects on cardiovascular and on fatty liver disease, statins are highly advisable in the management of NAFLD patients regardless of their lipid profile. We recommend them in NASH even in the presence of normal lipids. In compensated cirrhosis statins remain safe; in these patients twenty milligram daily dose is associated with less myopathy compared to 40 mg per day. Initiation of statins is not recommended in decompensated cirrhosis, hypothyroidism or kidney disease due to high risk of side effects notably myopathies. Continuation of statins in decompensated cirrhosis in those already on them remains controversial; we suggest dose reduction to 20 mg per day in this circumstance.
Insulin sensitizing agents: Pioglitazone, a Peroxisome proliferator-activated receptor (PPAR) gamma agonist is a potent insulin sensitizer, it preserves beta-cell function, reduces HbA1c, and reduces CV events and components of the metabolic syndrome.
A meta- analysis by Musso et al of 8 RCT of patients with NASH with and without T2 D, showed that pioglitazone at 35-45 mg daily and up to 24 months led to resolution of NASH. Of 101 patients studied by Cusi et al., 51% had resolution of NASH and improvement of fibrosis. However pioglitazone showed side effects that included weight gain of 2.7% and edema. These side effects are minimized with reduced doses. It is strongly advisable to avoid pioglitazone in individuals with bladder cancer, fractures and heart failure.
Glucagon like peptide 1 receptor agonists: Liraglutide and Semaglutide are FDA approved for the treatment of type 2 diabetes and obesity. They have shown additional beneficial effects on cardiovascular events and on resolution of NASH and fibrosis in the liver, and their use is encouraged in patients with and without type 2 diabetes.
When studied in NASH patients, in the LEAN trial, Liraglutide at a dose of 0.8 mg SQ daily given over 48 weeks, was associated with significant resolution of NASH and regression of fibrosis compare to placebo in patients with and without diabetes. Eligible patients with diabetes in the study, were well controlled on metformin or sulfonylurea and had a stable hga1c <9.
In a prospective randomized controlled trial, the combination of exercise with Liraglutide 3mg SQ daily was associated with the highest weight loss of 16% at one year than either form alone in addition to cardiometabolic benefits in patients with and without diabetes. Adverse events included abdominal pain and nausea, diarrhea, palpitations, gallbladder disease and pancreatitis. Another randomized controlled trial in patients who did not have diabetes showed a weight reduction of 5-10% which is a significant reduction compared to placebo at 56 weeks.
Semaglutide, a long acting GLP1 RA, was assessed in phase 2 double blinded clinical trial of 230 patients with NASH and fibrosis F2 - F3 who received 2 liver biopsies at enrollment and at 72 weeks. Semaglutide was given SQ daily at doses equivalent to 0.1 mg, 0.2 mg and 0.4 mg. Improvement in features of NASH was seen for all doses. The percentage of patients with NASH resolution and no worsening of fibrosis was significantly higher in the Semaglutide group at 0.4 mg in comparison to placebo (59% vs17%).
A phase 3 double blinded clinical trial of lifestyle intervention plus placebo versus Semaglutide 2.4mg SQ weekly was conducted to assess efficacy in reducing weight by at least 5% in individuals with BMI > 30 Kg/m2 or BMI > 27 Kg/m2 plus > 1 comorbidity but not diabetes for 68 weeks. Results showed an average weight loss of 14.9%, with 80% of participants achieving a weight loss of at least 5%, and 50% of participants achieved a weight loss of 15%. Approximately, a third lost 20% of baseline weight in addition to improvement of cardiometabolic parameters. Adverse events were nausea and gallbladder disorders. Semaglutide should not be used in patients allergic to it, with a personal or family history of medullary thyroid carcinoma, in patients with multiple endocrine neoplasia2, with pancreatitis or diabetic ketoacidosis.
Bariatric Intervention
Bariatric surgery (Roux-en-Y Gastric Bypass RNYGB, Sleeve or Gastric Band) is an option in individuals with NAFLD who are unable to achieve the intended weight loss by conservative means or lifestyle modifications. It is indicated when BMI is > 30 kg/m2 in the presence of T2D or for a BMI > 40 Kg/m2 due to its cardiometabolic benefits.
Studies in NASH have shown that bariatric surgery, regardless of type, significantly improved steatosis and to a lesser degree fibrosis when patients were followed up to 5 years. Weight loss of 25 Kg peaked at 2 years. Even though, death rates of 0.6% occurred within the first post- operative year, bariatric surgery is considered to be safe and associated with less major liver or cardiovascular outcomes when compared with the non-surgical group at 7 year follow up.
Reoperations were common, progression of fibrosis and complications occurred less <9%. Weight regain was reported between 3-35% depending on the type of surgery, and it is suggested to combine weight reduction surgery with lifestyle modification and medical therapy in order to achieve a sustained weight loss.
Bariatric surgery is not recommended in decompensated cirrhosis and should be considered very carefully in cirrhosis or advanced fibrosis, due to high risk of complications and liver decompensation. It should not be performed without the assistance of nutrition specialists due to protein loss and micronutrient deficiencies that can be grave.
There is limited evidence to support recommendation for endoscopic bariatric therapies in persons with NAFLD at this point.
Other considerations: Prevention of viral hepatitis by vaccinating against hepatitis A and B is recommended.
Futures therapies
Several medications, alone or in combination, or in combination with weight reduction interventions, targeted at regressing steatosis and or fibrosis are in phase 2 and 3 clinical trials and may show promise in the management of this severe epidemic. It is premature to discuss them at the current time, but it is worth listing a few of these therapies and they include: Cysteamine bitartrate (antioxidant, glutathione precursor), Cenicriviroc (anti TNF, antifibrotic), selonsertib and Simutuzumab (antifibrotic), Elafibrinor (PPARαδ insulin sensitizer), Termisartan and Losartan (ACE and ARB inhibitors).Tirzapetide ( a dual GIP-GLP1 RA in phase 2 clinical trials for its weight reduction effects) , Resmiteron (aTHR-β-selective agonist reduces hepatic fat fraction ), Aldafermin ( reduces liver fat content)
Steps in the evaluation of fatty liver
|
1 |
Abnormal liver enzymes plus fatty liver on imaging |
||||||||||||
|
2 |
Exclude alcohol and other conditions that can cause fatty liver disease |
||||||||||||
|
3 |
HgbA1c, lipid panel, CRP, TSH |
||||||||||||
|
4 |
Referral for cardiac risk assessment and prevention |
||||||||||||
|
5 |
Risk stratification for developing cirrhosis |
||||||||||||
|
6 |
Lifestyle modification for all: |
||||||||||||
|
7 |
Risk
|
||||||||||||
Suggested readings
Epidemiology and natural history
- Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. 2014 Jun; 59(6):2188-95.
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. 2016 Jul;64(1):73-84..
- Allen AM, Therneau TM, Larson JJ, et al. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 year-community study. Hepatology. 2018;67(5):1726-36.
- Arun J Sanyal, Mark L Van Natta, Jeanne Clark, Brent A Neuschwander-Tetri , AnnaMae Diehl, Srinivasan Dasarathy et al, NASH Clinical Research Network (CRN). Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. New Engl J Med 2021: Oct 21;385(17):1559-1569
- Adam C. Sheka, Oyedele Adeyi, Julie Thompson, Bilal Hameed,Peter A. Crawford, MD, PhD; Sayeed Ikramuddin, MD, MHA. Nonalcoholic Steatohepatitis.A Review. JAMA. 2020 Mar 24;323(12):1175-1183
- Brunt EM, Wong VW, Nobili V, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080
- Singal AK, Hasanin M, Kaif M, et al. Nonalcoholic Steatohepatitis is the Most Rapidly Growing Indication for Simultaneous Liver Kidney Transplantation in the United States. Transplantation 2016;100:607-12
- Doycheva I, Watt KD, Rifai G, Abou Mrad R, Lopez R, Zein NN, Carey WD, Alkhouri N. Increasing Burden of Chronic Liver Disease Among Adolescents and Young Adults in the USA: A Silent Epidemic. Dig Dis Sci. 2017 May;62(5):1373-1380. doi: 10.1007/s10620-017-4492-3. Epub 2017 Feb 13.
- Kotlyar DS, et al. Recurrence of diseases following orthotopic liver transplant Am J Gastroenterol 2006; 101:1370-1378
- McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004 Aug;8 (3):521-33, viii.
- Zobair M Younossi, Pegah Golabi, Leyla de Avila, James Minhui Paik, Manirath Srishord, Natsu Fukui, Ying Qiu, Leah Burns, Arian Afendy, Fatema Nader . The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J hepatol 2019 Oct;71(4):793-801.
- Selvaraj imaging in patients with NAFLD: A systematic review and meta-analysis. J Hepatol. 2021 Oct;75(4):770-785.2021 EA, Mózes FE, Jayaswal ANA, Zafarmand MH, Vali Y, Lee JA, Levick CK, Young LAJ, Palaniyappan N, Liu CH, Aithal GP, Romero-Gómez M, Brosnan MJ, Tuthill TA, Anstee QM, Neubauer S, Harrison SA Harrison. Diagnostic accuracy of elastography and magnetic resonance
- Williams CD, Stengel J, Asike MI et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy. Gastroenterol 2011; 140: 124-131
- Daniel O Huang, Hashem-B El-serag and Rohit Loomba. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention
- Michael Delicce, Joseph Mauch, Abel Joseph, Ruishen Lyu, Heather Kren, Rose Bartow, Donna Ferchill, Maan Fares, Jamile’ Wakim-Fleming. Cardiac Risk Factors Limiting Survival to Liver Transplantation in Patients with Nonalcoholic Fatty Liver Disease..Wrld Journal of Hepatology publication in Press
Pathophysiology
- NAFLD 2020, Gastroenterology, special issue 158(7).May 2020
- Lee DH, Cho EJ, Bae JS, Lee JY, Yu SJ, Kim H, Lee KB, Han JK, Choi BI. Accuracy of Two-Dimensional Shear Wave Elastography and Attenuation Imaging for Evaluation of Patients With Nonalcoholic Steatohepatitis Clin Gastroenterol Hepatol. 2021 Apr;19(4):797-805.e7
- Zarrinpar A, Chaix A, Panda : Daily eating patterns and their impact on health and disease: Trends Endocrinol Metabol 2016;27: 69-83
- Anand R Saran 1 , Shravan Dave 1 , Amir Zarrinpar. Circadian Rhythms in the Pathogenesis and Treatment of Fatty Liver Disease Gastroenterology . 2020 May;158(7):1948-1966.
Diagnosis
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network.Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology . 2005 Jun;41(6):1313-21.
- Loomba R, Adams LA.Advances in non-invasive assessment of hepatic fibrosis. 2020 Jul;69(7):1343-1352.
- Runge JH, Smits LP, Verheij J, Depla A, Kuiken SD, Baak BC, Nederveen AJ, Beuers U, Stoker J. MR Spectroscopy-derived Proton Density Fat Fraction Is Superior to Controlled Attenuation Parameter for Detecting and Grading Hepatic steatosis. 2018 Feb;286(2):547-556
- StokerEddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019 May;156(6):1717-1730.
- Mohammad S. Siddiqui,1Raj Vuppalanchi,1Mark L. Van Natta,3Erin Hallinan,3Kris V. Kowdley,4Manal Abdelmalek,5Brent A Neuschwander-Tetri,6Rohit Loomba,7Srinivasan Dasarathy,8Danielle Brandman,9Edward Doo,10James A. Tonascia,3David E. Kleiner, MD,11Naga Chalasani,2,¶Arun J. Sanyal,1,¶ and for the NASH Clinical Research Network. Vibration-controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019 Jan; 17(1): 156–163.e2.
- de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-974
Management
- Thomas C, Day CP, Tenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol 2012 Jan;56(1):255-66.
- Ryan MC, Isiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol 2013;59:138-143.
- Hayat, U., Siddiqui, A.A., Okut, H., Afroz, S., Tasleem, S., Haris, A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studiesAnnals of Hepatology. Volume 20, January–February 2021
- Sanyal AJ, Chalasani MB, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 362:1675- 1685.
- Jung Il Lee , Hyun Woong Lee , Kwan Sik Lee , Hye Sun Lee 3 , Ju-Young Park. Effects of Statin Use on the Development and Progression of Nonalcoholic Fatty Liver Disease: A Nationwide Nested Case-Control Study. Am J Gastroenterol 2021 Jan 1;116(1):116-124.
- Vilar-Gomez E, Vuppalanchi R, Gawrieh S, Ghabril M, Saxena R, Cummings OW, Chalasani N. Vitamin E Improves Transplant-Free Survival and Hepatic Decompensation Among Patients With Nonalcoholic Steatohepatitis and Advanced Fibrosis. Hepatology . 2020 Feb;71(2):495-509.
- Rebecca G. Kim, MD, MS,1 Rohit Loomba, MD, MHSc,2,3,4 Larry J. Prokop, MLS,5 and Siddharth Singh, MD, MS. Statin Use and Risk of Cirrhosis and Related Complications in Patients with Chronic Liver Diseases: a Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017 Oct; 15(10): 1521–1530.e8.
- Chalasani N, Younossi Z, Lavineet J, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guideline from the American Association for the Study of Liver Diseases. Hepatology 2018 Jan;67(1):328-357
- European Association for the Study of the Liver, European Association for the Study of Diabetes, and European Study for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Journal of Hepatology 2016. Volume 64, Issue 6, 1388-1402.
- Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. Lundgren JR, Janus C, Jensen SBK, Juhl CR, Olsen LM, Christensen RM, Svane MS, Bandholm T, Bojsen-Møller KN, Blond MB, Jensen JB, Stallknecht BM, Holst JJ, Madsbad S, Torekov SS.Lundgren JR, et al.N Engl J Med. 2021 May 6;384(18):1719-1730
- A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. Philip N. Newsome, M.B., Ch.B., Ph.D., Kristine Buchholtz, M.D., Ph.D.,Kenneth Cusi, M.D., Martin Linder, M.Sc., Takeshi Okanoue, M.D., Ph.D., Vlad Ratziu, M.D., Ph.D., Arun J. Sanyal, M.D., Anne-Sophie Sejling, M.D., Ph.D., and Stephen A. Harrison, M.D, N Engl J Med 2021; 384:1113-1124
- Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF; STEP 1 Study Group.Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Mar 18;384(11):989-1002.
- Majzoub AM, Nayfeh T, Barnard A, Munaganuru N, Shravan Dave, Siddharth Singh, Mohammad Hassan Murad , Rohit Loomba: Systematic review and network meta-analysis: comparative efficacy of pharmacologic therapies for fibrosis improvement and resolution of NASH. Aliment Pharmacol Ther. 2021 Aug 25
- Lassailly G, Caiazzo R, Ntandja-Wandji LC, et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159(4):1290-1301.e5
- Raluca Pais, Judith Aron-Wisnewsky, Pierre Bedossa, Maharajah Ponnaiah, Jean-Michel Oppert, Jean-Michel Siksik, Laurent Genser, Frederic Charlotte, Dominique Thabut, Karine Clement, Vlad Ratziu Persistence of severe liver fibrosis despite substantial weight loss with bariatric surgery. Hepatology 2022 Jan 25
- Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, Abdelmalek MF, Harrison SA, Loomba R, Mantzoros CS, Bugianesi E, Eckel RH, Kaplan LM, El-Serag HB, Cusi K. et al. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease 2021 Nov;161(5):1657-1669
- Aminian A, Al-Kurd A, Wilson R, Bena J, Fayazzadeh H, Singh T, Albaugh VL, Shariff FU, Rodriguez NA, Jin J, Brethauer SA, Dasarathy S, Alkhouri N, Schauer PR, McCullough AJ, Nissen SE.. Association of Bariatric Surgery With Major Adverse Liver and Cardiovascular Outcomes in Patients With Biopsy-Proven Nonalcoholic Steatohepatitis. 2021 Nov 23;326(20):2031-2042.
- Arterburn DE, Telem DA, Kushner RF, Courcoulas AP.Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA. 2020 Sep 1;324(9):879-887.