Background
Sandra Rodriguez, MD
William D. Carey, MD
A multitude of viruses may cause hepatic inflammation but 5, designated A, B, C, D, and E primarily infect the liver and produce hepatitis as their primary clinical manifestation (Table 1). Historically, hepatitis E virus (HEV) has been known to cause acute hepatic inflammation almost exclusively. Usually, in a self-limiting fashion that may infrequently progress to fulminant hepatitis. Transmission is predominantly through the fecal-oral route, through contaminated water. In such ways, it has been known to cause epidemics in high prevalence areas.
Table 1. Comparison of the worldwide burden of hepatitis
| Global† Acute |
Chronic |
United States Acute |
Chronic |
|
|---|---|---|---|---|
| HAV | 1/4 | N/A | 21000 | N/A |
| HBV | 4 | 240 | 38000 | 1.4 |
| HCV | 3-4 | 150 | 16000 | 3.9 |
| HEV | 20/3* | Unknown | Unknown | Unknown |
† All numbers are reported in millions.
* 20 million new infections and 3 million cases of acute hepatis.
Hepatitis data can be found at www.who.int/topics/hepatitis/factsheets/en/.
In none endemic areas the prevalence of the disease used to be practically nil. However, in recent years there has been a major increase in the number of cases. Recent seroprevalence reports estimate that about one-third of the world population is positive for anti-HEV IgG antibody; a statistic that arguably makes HEV the new leading cause of acute hepatitis in the world and a major universal public health burden.
Definition
HEV is a small, spherical, non-enveloped virus that is 32 to 34 nm in diameter. It carries a single stranded RNA genome that is 7.2 kB in length and contains 3 open reading frames (ORFs). ORF1 encodes for messenger RNA which codes for non-structural proteins that are required for viral replication. ORF2 encodes for the viral capsid that is responsible for activation of the immune system. ORF3 encodes for small non-structural proteins that contribute to HEV replication and pathogenesis. The genome contains short non-coding regions at the 5' and 3' ends, and in between the ORFs which are discontinuous and partially overlapping (Figure 1).
Figure 1. Hepatitis E Virus Generic Map
| 2kB | 7.5kB | ||||
| OrF-1 | ORF-3 | OrF-2 | |||
| Melthyltransferase | Cysteine protein | RNA helicase | RNA polymerase | Phosophoprotein | Capsid protein |
The virus was previously recognized as part of the Calciviridae family in the genus Calcivirus, but recently it was reclassified into the Hepeviridae family in the Hepevirus genus. Namely, the Hepeviridae family consists of 2 species: first the mammalian HEV strains, which affects humans and pigs typically; and second the avian strain, which is accountable for big liver-spleen disease in chicken and can also affect other birds.13 Mammalian strains are grouped into 4 different genotypes, 1 through 4, each with several subtypes and approximately about an 80% homology among genotypes.
Different genotypes have marked differences in epidemiology. Genotype 1 and 2 are highly prevalent in endemic areas causing epidemics among humans. Both have high mortality rates in pregnant women, a trait also shared by genotype 4. Genotype 3 is most prevalent in developed countries. It has been shown to be transmitted by animal contact or by consuming contaminated aliments. It is typically seen in individuals aged over 40 years and is known to cause severe disease in immunocompromised individuals in whom it can become chronic. Pigs commonly harbor genotypes 3 and 4.
Avian strains have a 50% homology with the mammalian strains. There are 3 distinct genotypes: Genotype 1 was identified in Australia, genotype 2 is most common in the US, and genotype 3 was identified in Europe and China. Avian strains are similar to swine strains since they have the ability to cross infect closely related species (ie, turkeys). Experimentally they have not been shown to infect monkeys, and so they probably less likely to infect humans.
Epidemiology
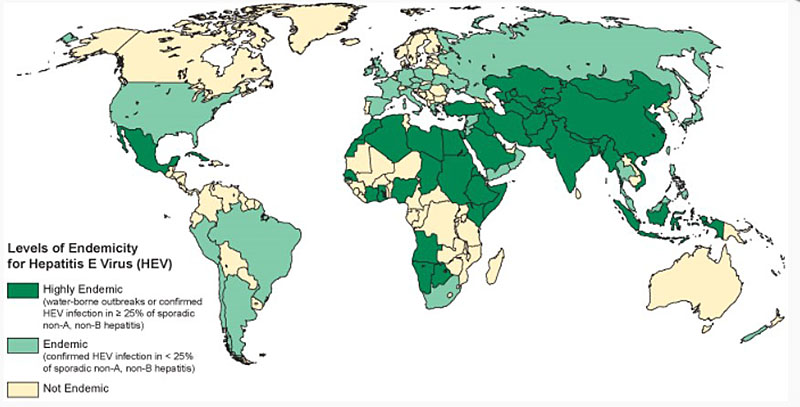
Figure 2: Geographic distribution of hepatitis E-outbreaks or confirmed infection in more than 25% of cases of sporadic non-ABC hepatitis.
Global HEV prevalence is on the rise. Approximately 2 billion people worldwide are positive for anti-HEV IgG antibody. The World Health Organization (WHO) reported that approximately 20 million people are infected yearly, and of those cases approximately 3 million progress to an acute hepatitis syndrome. There are about 70, 000 deaths related to HEV worldwide per year. Genotype 1 is endemic to Central and Southeast Asia, Africa and Central America where it is known to cause epidemics. The virus has also been isolated in low endemic areas among travelers from high prevalence regions. Genotype 2 was first identified in Mexico, and subsequently has been reported from Nigeria and Chad. Genotype 2 is not as commonly a cause of epidemic compared with genotype 1. Genotypes 3 and 4 are common in the swine population in developed countries; and are emerging as significant causes of human infection. The first case of a human infected with genotype 3 was identified in the United States and subsequently in Europe, Australia, and Japan. Genotype 4 has been found in sporadic cases in Vietnam, China, and Taiwan. (Figure 2).
The highest seroprevalence of HEV is seen in countries of low socioeconomic status and poor sanitation; reportedly 20% to 40%.4 Outbreaks happen commonly during rainy season, when potable water is contaminated by flooding18 or with disposal of fecal waste into usable water sources. In urban underdeveloped areas, outbreaks commonly occur through water conduits that pass through soil that is contaminated with human excrements. Lastly, there is also a large proportion of sporadic HEV cases in endemic areas. The mode of transmission or risk factors is not known and whether animal spread plays a role remains a mystery.
In developed countries, human HEV was considered uncommon, with a prevalence of less than 1%. However, rates of anti HEV antibody within the general population have increased significantly; 2% in Europe and 1%-3% in the United States, with up to 20% prevalence in certain high-risk groups. The majority of cases are sporadic, and high risk populations include farmers, animal butchers, veterinarians, persons that handle animal products or consume of undercooked meat. As such, animals compose a key reservoir for HEV infection in the first world, and probably accounts for increase in prevalence.
Sporadic cases are more common in patients aged 60 years or older, with a 3-to-1 male-to-female ratio. The primary mode of transmission is foodborne from ingestion of contaminated products or from direct contact with an infected animal. In the US, human HEV strains have been genetically isolated from pigs, chickens, rabbits, rats, and fish.12,28. The highest prevalence is among the swine population with genotype 3; genotype 4 has been identified in swine in other countries.
Ming et al. found HEV to be ubiquitous in the population of swine aged older than 3 months herds throughout the Midwestern US, with a prevalence of 80% to 100% in some groups. In the same study, it was noted that the ORF2 of swine HEV had a 90% amino acid sequence similarity with human HEV strains and that ORF3 had 80% amino acid sequence similarity with human HEV strains prevalent in the United States. Feagins et al reported that 11% of the commercial pig livers sold in local grocery stores are contaminated by infectious HEV. These statistics probably account for the 21% reported prevalence of anti-IgG HEV in the US. Similar statistics have been reported in Europe and Japan where sporadic cases have been directly linked to undercooked pig livers.
Lastly, evidence also exists to implicate shellfish as a foodborne source of infection and a public health concern. This is made evident by reported outbreaks of genotype 3 among 33 cruise ship passengers who consumed contaminated shellfish. In addition, 2 cases of HEV were identified in the United Kingdom in 2005, both found to be related to consumption of shellfish. The mechanism of contamination seems to be related to agricultural waste spills that mix with shellfish harvesting waters.
Pathophysiology and Natural History
In an acute infection the incubation period ranges from 15 to 60 days, through which viremia arises. Anti-HEV antibody (IgM) appears soon after the onset of the clinical infection. Anti-HEV IgG appears soon after that and can remain detectable for as long as 20 months (Figure 3). HEV RNA is detected in stool as early as 1 week before the onset of clinical illness and persists for 1 to 2 weeks afterward, during which stools are highly infectious. (Figure 3 and 4)
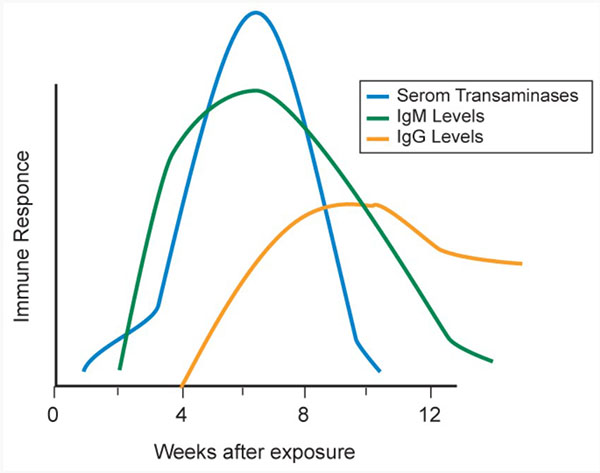
Figure 3. HEV Serologic Course
Figure 4. Hepatitis E particles in stool. Hepatitis E virus (HEV), the majore causative agent of enterically transmitted non-A, non-B hepatitis worldwide, is a spherical, nonenveloped, single-stranded RNA virus that is approximately 32 to 34 nm in diameter. From the Centers for Disease Control: Hepatitis E Virus, 2007.
Similar to hepatitis A, HEV is most often a self-limited disease; immunity develops, and no second attack occurs. The disease is often cholestatic, with elevated bilirubin and alkaline phosphatase levels. On average, it occurs more commonly in the older population and severe disease, including fulminant hepatitis and death, occur more often in HEV than in other types of hepatitis. Mortality rates range from 0% to 10%, but it is usually less than 1%. In pregnant women, infection has been associated with development fulminant liver failure and mortality can reach 25%.
Modes of transmission include: fecal-oral route, food borne, from exposure to infected animals, via blood transfusion, solid organ transplantation, and lastly via vertical transmission. Prolonged viremia and fecal shedding are uncommon. However, in developed countries progression to chronic disease and cirrhosis has been reported in older patients, those with pre-existing chronic liver disease, and the immunosuppressed.
Signs and Symptoms
Clinical presentation can range from asymptomatic infection to constitutional and gastrointestinal symptoms that cannot be differentiated from other types of viral hepatitis. The onset of symptoms tends to be abrupt and fever is uncommon. Prodromal symptoms improve or disappear with the onset of jaundice, although anorexia, malaise, right upper quadrant pain and weakness can persist. In most reported epidemics, jaundice is present in 90% to 100% of cases, suggesting that human HEV may be more likely than hepatitis A, B, or C to produce jaundice. Most infections resolve spontaneously and the case mortality rate is very low.
Sporadic cases present similarly, though are less likely to develop into jaundice. Most patients never manifest sings of infection; except in the older population in which progression to an acute hepatitis is common. As such, it is believed that HEV remains sub clinical in women and younger persons. They has been associated with a number of extra hepatic manifestations, including polyarthritis, pancreatitis, pure red cell aplasia and aplastic anemia. Neurologic complications such as peripheral neuropathies, polyradiculopathy, Bell's palsy, ataxia, and Guillain-Barré syndrome have been reported. Acute on chronic disease, in which patients with underlying chronic liver disease progress rapidly to decompensated liver failure after being infected with HEV has been observed as well. Progression to cirrhosis is evident due to persistently elevated serum aminotransferases and progression of liver disease to fibrotic stages.
Diagnosis
Acute HEV infection should be suspected in patients who live in endemic areas or return from high prevalence countries and develop constitutional symptoms, jaundice, and acute elevation of serum transaminases. Sporadic infection should be excluded in patients with acute hepatitis who are at high risk due to close contact with animals or from consumption of undercooked meat. Diagnosis is made mainly through serologies although there is no standardized testing protocol available. Worldwide, all serologic tests are of limited value in diagnosis since their sensitivity and specificity vary greatly.
In the US, serologic testing is only available in commercial research laboratories; however, no testing has been approved by the Food and Drug Administration (FDA). If serologic testing is indicative of an acute infection polymerase chain reaction (PCR) assays for HEV RNA on serum and/or stool should be performed as a confirmatory test. One major drawback of PCR is that results can be negative the setting of an acute HEV infection, since the viremic phase is brief, hence, a negative PCR does not exclude the diagnosis. Similarly, in immunosuppressed patients serologic testing may be negative in the setting of a weakened immune system.
In general, if HEV is suspected testing should include an initial set of serologies followed by confirmatory PCR in those with normal immune systems. In immunocompromised patients PCR is the most reliable test for diagnosis.53 If there is progression to chronic liver disease, PCR should be used to assess response to available experimental treatments.
Treatment and Prevention
Treatment of acute infection is supportive. Patients who can maintain hydration and nutrition can be treated at home; those with severe symptomatic disease can require hospitalization. Persons traveling to endemic areas should be aware of the virus and avoid water, uncooked food, and contact with contaminated substances. Common sense dictates that societal commitment to good public health, clean water supplies, and personal sanitation will reduce the incidence of HEV.
In developed countries, cooking pork meat appropriately, by heating to 71°C or 160°F for at least 20 and avoiding raw shellfish can prevent sporadic HEV infections. These measures should be strictly followed, especially in those who are at high risk for acquiring the disease and/or progressing to chronic liver failure.
Case reports have shown resolution of infection in some patients by immune reconstitution. Experimental treatment with Interferon-Ribavirin based therapy has been used in patients who develop chronic disease, and has shown to attain viral clearance in most cases.
HEV is preventable by vaccination. HEV239 (Hecolin) is a recombinant HEV vaccine against genotype 1 and 4 that has shown to have more than 95% protection against the virus and to be safe in pregnancy. This vaccine is now available in China. In the US there is no currently approved form of vaccination.
Summary and Recommendations
- HEV is an RNA virus that infects humans through oral contamination of water.
- It is seen principally in Southeast and Central Asia, the Middle East, and Africa, or in travelers returning from endemic areas.
- Spread is both from person to person and through animal intermediaries, such as swine.
- There is an increase in seroprevalence in developed countries owed to its large animal reservoir.
- Illness caused by HEV might be asymptomatic, but is much more likely to produce jaundice than other forms of acute viral hepatitis.
- The case-fatality rate is 1.7%, but pregnant women seem especially susceptible to severe disease and a fatal outcome.
- In developed countries sporadic HEV infection is not uncommon.
- HEV should be considered in persons at high risk (ie, men older than 60 years of age, farmers, animal butchers, veterinarians, persons that handle animal products, consumers of undercooked meat and immunosuppressed patients).
- Sporadic cases have been associated with extrahepatic manifestations, acute on chronic liver failure in those with underlying liver disease, and progression to chronic disease in males older than 60 years of age and the immunosuppressed population (ie, HIV, status post organ transplant, and those receiving chemotherapy).
- Management of acute infection is mostly supportive, similar to other forms of acute viral hepatitis.
- Management of chronic infection is still experimental, but effective viral clearance seems to be achieved through interferon-ribavirinn based therapy.
- A vaccine is available as of 2012 in China that appears to confer close to 100% protection again HEV and it is safe in pregnancy.
- No vaccination is yet available in the US.
Suggested Reading
- "Hepatitis E in the United States" an NIH workshop available at https://www.niddk.nih.gov/news/meetings-workshops/2012/hepatitis-e-in-the-united-states-an-nih-research-workshop.
- Global Prevalence of Hepatitis E Virus Infection and Susceptibility available at https://iris.who.int/handle/10665/70513
- Velázquez O, Stetler HC, Avila C, et al. Epidemic transmission of enterically transmitted non-A, non-B hepatitis in Mexico, 1986-1987. JAMA 1990;263:3281-3285.
- Mushahwar IK. Hepatitis E virus: molecular virology, clinical features, diagnosis, transmission, epidemiology, and prevention. J Med Virol 2008;80:646-658.
- Khuroo MS, Rustgi VK, Dawson GJ, et al. Spectrum of hepatitis E virus infection in India. J Med Virol 1994;43:281-286.
- World Health Organization. Viral Hepatitis Report by the Secretariat. A62/22. who.int/gb/ebwha/pdf_files/A62/A62_22-en.pdf.
- Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012;55(4):988-97.
- Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol 2008;48(3):494-503.
- Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med 2012;367:1237-1244.
- Ahmad I, Holla RP, Jameel S. Molecular virology of hepatitis E virus. Virus Res 2011;161(1):47-58. Epub 2011 Feb 21.
- Chandra V, Kalia M, Hajela K, Jameel S. The ORF3 protein of hepatitis E virus delays degradation of activated growth factor receptors by interacting with CIN85 and blocking formation of the Cbl-CIN85 complex. J Virol 2010;84(8):3857-3867. Epub 2010 Feb 3.
- Chandra V, Taneja S, Kalia M, Jameel S. Molecular biology and pathogenesis of hepatitis E virus. J Biosci 2008;33:451-464.
- Teshale EH, Hu DJ. Hepatitis E: Epidemiology and prevention. World J Hepatol 2011;3:285-291.
- Purcell RH, Engle RE, Rood MP, et al. Hepatitis E virus in rats, Los Angeles, California, USA. Emerg Infect Dis 2011;17: 2216-2222.
- Haqshenas G, Shivaprasad HL, Woolcock PR, Read DH, Meng XJ. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J Gen Virol 2011;82:2449-2462.
- Labrique AB, Zaman K, Hossain Z, et al. Epidemiology and risk factors of incident hepatitis E virus infections in rural Bangladesh. Am J Epidemiol 2010;172:952-961.
- Gomatos PJ, Monier MK, Arthur RR, et al. Sporadic acute hepatitis caused by hepatitis E virus in Egyptian adults. Clin Infect Dis 1996; 23:195-196.
- Abe K, Li TC, Ding X, W, et al. International collaborative survey on epidemiology of hepatitis E virus in 11 countries. Southeast Asian J Trop Med Public Health 2006;37:90-95.
- Kuniholm MH, Purcell RH, McQuillan GM, et al. Epidemiology of hepatitis E virus in the United States: Results from the third National Health and Nutrition Examination Survey, 1988-1994. J Infect Dis 2009;200:48-56.
- Viswanathan R. A review of the literature on the epidemiology of infectious hepatitis. Indian J Med Res 1957;45:145-155.
- Coursaget P, Buisson Y, N'Gawara MN, Van Cuyck-Gandre H, Roue R. Role of hepatitis E virus in sporadic cases of acute and fulminant hepatitis in an endemic area (Chad). Am J Trop Med Hyg 1998;58:330-334.
- Das K, Agarwal A, Andrew R, Frösner GG, Kar P.. Role of hepatitis E and other hepatotropic virus in aetiology of sporadic acute viral hepatitis: a hospital based study from urban Delhi. Eur J Epidemiol 2000;16:937-940.
- Labrique AB, Kuniholm MH, Nelson KE. The global impact of Hepatitis E—New horizons for an emerging virus. In: Scheld WM, Murray BE, Hughes JM, editors. Emerging Infections. 9th Edition. Virginia: ASM Press; 2010.
- Yazaki Y, Mizuo H, Takahashi M, et al. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol 2003;84: 2351-2357.
- Mizuo H, Yazaki Y, Sugawara K, et al. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J Med Virol 2005;76: 341-349.
- Chau TN, Lai ST, Tse C, et al. Epidemiology and clinical features of sporadic hepatitis E as compared with hepatitis A. Am J Gastroenterol 2006;101:292-296.
- Dalton HR, Stableforth W, Thurairajah P, et al. Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol 2008;20:784-90.
- Sainokami S, Abe K, Kumagai I, et al. Epidemiological and clinical study of sporadic acute hepatitis E caused by indigenous strains of hepatitis E virus in Japan compared with acute hepatitis A. J Gastroenterol 2004;39:640-648.
- Feagins AR, Opriessnig T, Guenette DK, Halbur PG, Meng XJ. Detection and characterization of infectious hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J Gen Virol 2007; 88: 912-917.
- Cossaboom CM, Córdoba L, Dryman BA, Meng XJ. Hepatitis E virus in rabbits, Virginia, USA. Emerg Infect Dis 2011;17:2047-2049.
- Zheng Y, Ge S, Zhang J, et al. Swine as a principal reservoir of hepatitis E virus that infects humans in eastern China. J Infect Dis 2006;193:1643-1649.
- Meng XJ, Purcell RH, Halbur PG, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA 1997;94:9860-9865.
- Meng XJ. From barnyard to food table: The omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res 2011;161:23-30.
- Sadler GJ, Mells GFG, Shah NH, et al. UK acquired hepatitis E─An emerging problem? J Med Virol 2006;78: 473-475.
- Chandra NS, Sharma A, Malhotra B, Rai RR. Dynamics of HEV viremia, fecal shedding and its relationship with transaminases and antibody response in pa- tients with sporadic acute hepatitis E. Virol J 2010;7:213.
- Hamid SS, Jafri SM, Khan H, et al. Fulminant hepatic failure in pregnant women: acute fatty liver or acute viral hepatitis? J Hepatol 1996;25:20-27.
- Purcell RH (1996). Hepatitis E virus. In Fields Virology, 3rd edn, pp. 2831-2843. Edited by Fields BN, Knipe DM, Howley PM. Philadelphia, PA: Lippincott-Raven.
- Boxall E, Herborn A, Kochethu G, et al. Transfusion-transmitted hepatitis E in a 'non-hyperendemic' country. Transfus Med 2006;16:79-83.
- Matsubayashi K, Kang JH, Sakata H, et al. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion 2008;48:1368-1375.
- Xu C, Alter HJ. An assessment of HEV prevalence and risk in U.S. blood donors and recipients. (https://pubmed.ncbi.nlm.nih.gov/23829163/), p 23. (Accessed December 3, 2012).
- Monga R, Garg S, Tyagi P, Kumar N. Superimposed acute hepatitis E infection in patients with chronic liver disease. Indian J Gastroenterol 2004;23: 50-52.
- Kamar N, Garrouste C, Haagsma EB, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011;140: 1481-9.
- Ollier L, Tieulie N, Sanderson F, et al. Chronic hepatitis after hepatitis E virus infection in a patient with non-Hodgkin lymphoma taking rituximab. Ann Intern Med 2009;150:430-431.
- Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med 2009;361:1025-1027.
- Jagjit Singh GK, Ijaz S, Rockwood N, et al. Chronic hepatitis E as a cause for cryptogenic cirrhosis in HIV. J Infect 2011 Dec 6. Epub ahead of print. PMID: 22166370.
- Kenfak-Foguena A, Schöni-Affolter F, Bürgisser P, et al. Data Center of the Swiss HIV Cohort Study, Lausanne, Switzerland. Hepatitis E virus seroprevalence and chronic infections in patients with HIV, Switzerland. Emerg Infect Dis 2011;17(6):1074-1078.
- Kaba M, Richet H, Ravaux I, et al. Hepatitis E virus infection in patients infected with the human immunodeficiency virus. J Med Virol 2011;83(10):1704-1716.
- Keane F, Gompels M, Bendall R, et al. Hepatitis E virus coinfection in patients with HIV infection. HIV Med 2012;13(1):83-88.
- Crum-Cianflone NF CJ, Drobeniuc J, Weintrob A, et al. Infectious Disease Clinical Research Program HIV Working Group. Hepatitis E virus infection in HIV-infected persons. Emerg Infect Dis 2012;18(3):502-506.
- Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet 2012;379:2477-2488.
- Kamar N, Bendall RP, Peron JM, et al. Hepatitis E virus and neurologic disorders. Emerg Infect Dis 2011;17:173-179.
- Bendall R, Ellis V, Ijaz S, et al. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol 2010; 82(5):799-805.
- Mast EE, Alter MJ, Holland PV, Purcell RH. Evaluation of assays for antibody to hepatitis E virus by a serum panel. Hepatitis E Virus Antibody Serum Panel Evaluation Group. Hepatology 1998;27:857-861.
- Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis 2008;8(11):698-709.
- Enouf V, Dos Reis G, Guthmann JP, et al. Validation of single real-time TaqMan PCR assay for the detection and quantitation of four major genotypes of hepatitis E virus in clinical specimens. J Med Virol 2006;78:1076-1082.
- Barnaud E, Rogée S, Garry P, Rose N, Pavio N. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl Environ Microbiol 2012;78:5153-5159.
- Haagsma EB, Riezebos-Brilman A, van den Berg AP, Porte RJ, Niesters HG. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transpl 2010;16:474-477.
- Kamar N, Rostaing L, Abravanel F, et al. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis 2010;50:e30-3.
- Mallet V, Nicand E, Sultanik P, et al. Brief Communication: case reports of ribavirin treatment for chronic hepatitis E. Ann Intern Med 2010;153:85-89.
- Zhu FC, Zhang J, Zhang XF, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomized, double-blind placebo-controlled, phase 3 trial. Lancet 2010;376:895-902.