Epidemiology
Liver disease related to hepatitis B remains an important public health concern and a major cause of morbidity and mortality. It also presents a common challenging problem for practicing physicians.
Hepatitis B is found throughout the world, but its prevalence varies greatly; it is especially high in Asia, sub-Saharan Africa, and the South Pacific, as well as in specific populations in South America, the Middle East, and the Arctic. Prevalence in the United States varies, based on the population makeup, including the extent of the immigrant population from endemic areas, and on risk factors and behavior, such as the prevalence of intravenous drug use and homosexual practices. Public health agencies estimate that there are about 1.25 million people infected in the United States, but 2 billion people infected worldwide, with approximately 5% of the world's population (or 350 million people) being carriers of chronic hepatitis B. In a typical year, 70,000 Americans become infected with chronic hepatitis B virus (HBV), and approximately 5000 patients with chronic hepatitis B die of complications caused by the disease. Worldwide, chronic hepatitis B is the tenth leading cause of death.
Hepatitis B was first discovered in 1963 by Dr. Baruch Blumberg and colleagues, who identified a protein (the “Australia antigen” that reacted to antibodies from patients with hemophilia and leukemia. The association of this protein with infectious hepatitis was discovered 3 years later by several investigators, and the virus was specifically seen by electron microscopy in 1970.
HBV is a double-stranded hepatotropic DNA virus belonging to the family Hepadnaviridae. The virus infects only humans and some other nonhuman primates. Viral replication takes place predominantly in hepatocytes and, to a lesser extent in the kidneys, pancreas, bone marrow, and spleen. The viral genome is 3.2 kb in length and possesses four partially overlapping open-reading frames that encode various antigens. The intact virion is a spherical double-shelled particle with an envelope of hepatitis B surface antigen (HBsAg), an inner nucleocapsid of core antigen (HBcAg), and an active polymerase enzyme linked to a single molecule of double-stranded HBV DNA. Significant variability of the nucleotide sequence exists, and the virus can be subdivided into eight different genotypes, based on the degree of variation. The clinical importance of these is still uncertain, however.
Immunization
Effective vaccines for HBV prevention have been available in the United States since 1982. Hepatitis B vaccine has been described as the first effective anticancer vaccine, and its use has been promoted by the World Health Organization as routine care worldwide since 1997. Pre-exposure prophylaxis is highly successful. It achieves the dual goals of individual protection and herd immunity. Early strategies that targeted high-risk groups (e.g., adolescents and young adults) were not successful because of the lack of motivation of those in this age group to seek preventive care. Accordingly, universal vaccination for HBV is recommended for infants by the American Academy of Pediatrics since 1991, and has resulted in a 90% decline in new cases of hepatitis B in the U.S.
For unvaccinated individuals with a documented hepatitis B exposure, postexposure prophylaxis consists of a single dose of hepatitis B immunoglobulin (HBIG) injected intramuscularly, followed immediately by HBV vaccination.
Three recombinant hepatitis B vaccines are available in the United States, Engerix-B, Recombivax HB and Heplisav B.
Engerix B: For primary vaccination of those 19 years old and younger Engerix-B is given in a dose of 10mcg. The initial dose is repeated one month and six months for a total of three doses. For those 20 and older the dose is 20 mcg, and given according to the same schedule.
Reocombivax B: For Recombivax-HB the dosage for infants through age 10 is 5 mcg. The dosage is repeated in one and six months for a total of three doses. Adolescents (ages 11-15) may receive the same regimen OR receive 10 mc (the adult dose) repeated four to six months later for a total of two doses. For those 20 years or older, the dose is 20 mcg, repeated one and six months later for a total of three doses.
Heplisav B: The newest vaccine is Heplisav B. It has two advantages over the others: It requires only two immunizations (20 mcg) one-month apart. It appears to be more immunogenic, resulting in protection rates of 95%. In recognition of the substantial evidence of failure of adherence to multiple dose regimens, we recommend this vaccine two dose approach wherever possible.
Prevaccination screening for anti-HBs is not recommended except for adult patients who are likely to have been previously exposed, including those in high-risk groups (e.g., injection drug users, male homosexuals). Postvaccination testing for anti-HBs to document seroconversion is not routinely recommended, except for persons who are at risk for lack of response or continued exposure. Booster doses may be appropriate for high-risk patients if titers of anti-HBs fall below what is considered protective (10 IU/mL). The vaccine should be routinely administered to everyone younger than 18 years and to adults at risk of exposure. It should be given to neonates of HBV-infected mothers together with hyperimmune B globulin (HBIg).
Pathophysiology
The hepatitis B virus is not cytopathic. Liver injury in chronic hepatitis B is believed to be immunologically mediated. The severity and course of disease do not correlate with the level of virus in serum. Antigen-specific cytotoxic T cells are believed to play a role in the cell injury in hepatitis B, but they ultimately account for viral clearance. Specific cytokines produced by cytotoxic and other T cells also have antiviral effects, contributing to viral clearance without cell death. The lack of a vigorous and specific CD8+ cytotoxic T cell and CD4+ helper T cell response can allow chronic infection to develop. Recruitment of nonspecific T cells then results in low-level chronic inflammation and liver damage. Similarly, spontaneous seroconversion from HBeAg to anti-HBeAb during chronic hepatitis B is also immunologically mediated, as is suggested from the transient flare of disease that often immediately precedes clearance of HBeAg.
Natural History
Hepatitis B is spread parenterally, through intimate personal contact, and perinatally. Persons at risk include intravenous drug users, children of mothers with HBV, men who have sex with men, patients on hemodialysis, and those exposed to blood or blood products.
The incubation period ranges from 45 to 160 days. The acute illness is usually mild, particularly in children. Symptoms are non-specific. In adults, a few will become jaundiced; and hepatitis may be fulminant in 0.1% to 0.5%. Symptoms may last for a few days, except in more severe cases where symptoms, including profound fatigue, can last weeks.
Physical examination features are nonspecific but can include mild enlargement and slight tenderness of the liver, mild splenomegaly, and posterior cervical lymphadenopathy in 15% to 20% of patients. Fulminant disease (acute liver failure) manifests with a change in mental status (encephalopathy) and coagulopathy.
The risk of developing chronic infection defined as the persistence of HBsAg in the blood for longer than 6 months, depends on the age and immune function of the patient at the time of initial infection. Ninety percent of infected newborns, 30% of children younger than 5 years, and 10% of adults progress to chronic infection. Of these 15% to 40% develop hepatitis B-related sequelae in their lifetimes. Those with chronic infection spontaneously clear surface antigen at a rate of 0.5% per year. Those with chronic hepatitis B can develop extrahepatic manifestations, including arthralgias, mucocutaneous vasculitis, glomerulonephritis, and polyarteritis nodosa. The glomerulonephritis of hepatitis B occurs more commonly in children and is usually characterized by the nephrotic syndrome, with little decrease in renal function. Polyarteritis nodosa occurs primarily in adults and is marked by a sudden and severe onset of hypertension, renal disease, and systemic vasculitis with arteritis in the vessels of the kidneys, gallbladder, intestine, or brain. Rare extrahepatic manifestations are mixed essential cryoglobulinemia, pericarditis, and pancreatitis.
Diagnosis
There are 6 laboratory tests of importance in diagnosis of hepatitis B:
- Hepatitis B surface antigen (HBsAg)
- Antibody to Hepatitis B surface antigen (anti-HBs)
- Antibody to hepatitis B core antigen (anti HBc)
- Hepatitis B e antigen (HBeAg)
- Antibody to HBeAg: (anti HBe)
- Hepatitis B virus (HBV DNA)
Acute Infection
Throughout the world, the most common means of acquiring hepatitis B is from an infected mother. This is referred to as vertical transmission. Otherwise, infections most often occur during adolescence and adulthood, termed horizontal transmission. Acute hepatitis B is diagnosed by detecting both HBsAg and anti HBc (IgM) antibody. If testing is delayed, HBsAg may have disappeared. In that case the presence of anti HBc (IgM) alone is sufficient to make a diagnosis. IgM core antibodies are lost within 6 to 12 months of the onset of illness. Biochemically, serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels can increase to between 500 to 5000 U/L and fall after the acute phase of infection. Serum bilirubin levels seldom increase above 10 mg/dL, the alkaline phosphatase level is usually normal or only modestly elevated. Except in fulminant cases the prothrombin time is usually normal or mildly elevated (e.g., 1 to 3 seconds). The serum albumin level is normal or minimally depressed. Peripheral blood counts may show mild leukopenia, with or without relative lymphocytosis. Loss of HBsAg and the development of HBsAb signify recovery from the acute infection and the development of immunity (Fig. 1).
Figure 1 demonstrates the serologic time course of typical acute hepatitis B.
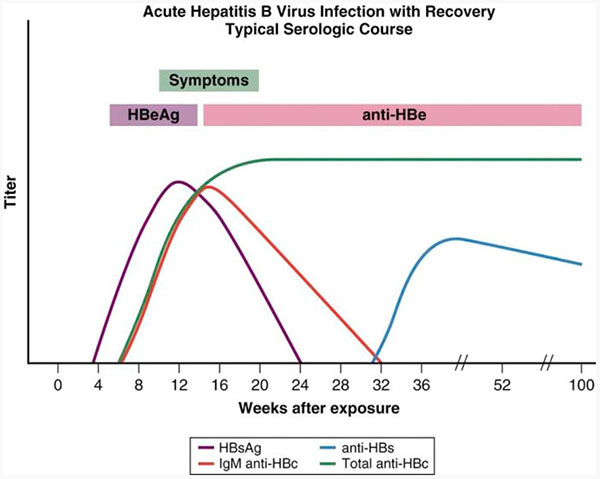
Figure 1: Serologic course of acute hepatitis B.
Chronic Infection
The serologic hallmarks of chronic hepatitis B is persistence of HBsAg and anti HBc, but absence of anti HBs. Those with chronic disease will have varying levels of circulating HBV DNA and liver inflammation. The serology of such chronic infections is shown in Figure 2.
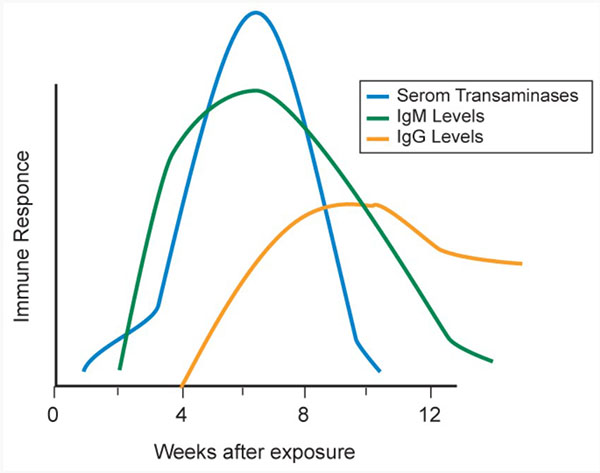
Figure 2. Serologic course of chronic hepatitis B.
A convenient way of interpreting laboratory testing results is shown in Box 1.
Box 1: Serologic Patterns for Hepatitis B
Immunity
Natural Exposure
- HBsAg negative
- HBcAb positive (or negative if distant in time)
- HBsAb positive
Vaccination
- HBcAb negative
- HBsAb positive
- HBsAg negative
Acute Infection
- IgM HBcAb positive
- HBsAb negative
- HBeAg may be positive or negative, depending on timing
- HBsAg positive
- HBV DNA-positive (usually)
Chronic Infection
- lgG HBcAb positive
- HBsAb negative
- HBsAg positive
- HBV DNA positive (usually)
HBcAb, hepatitis B core antibody; HBsAb, hepatitis B surface antibody; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; lg, Immunoglobulin.
© 2005 The Cleveland Clinic Foundation.
Chronic Hepatitis B
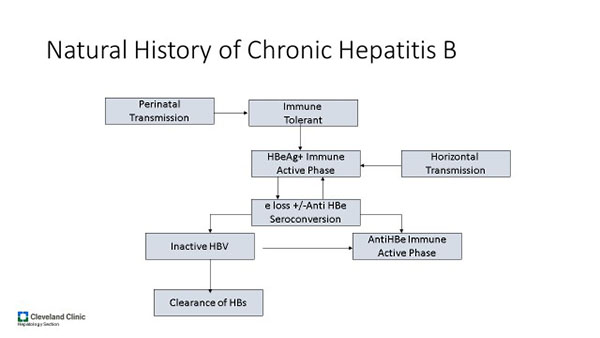
Chronic hepatitis B is defined as the persistence of HBsAg in serum for at least 6 months. The clinical course of hepatitis B varies considerably between individuals and in the same individual over time. The following describes recognized stages of chronic hepatitis B:
The terminology of varies states of chronic hepatitis B is confusing, arcane, and imperfect. Some individuals are difficult to classify. At the end of the day, we are most often interested in recognizing those with chronic hepatitis B who have, or might, develop progressive liver injury, and so who will benefit from anti-viral treatment. The amount of virus in serum is an imperfect predictor of those likely to have active liver disease. For example, the child with vertically acquired hepatitis B often have extremely high viral loads (Immune tolerant; Phase 1), yet normal liver tests and normal liver histology. The following classification developed by the European Association for the Study of Liver is a useful scheme.
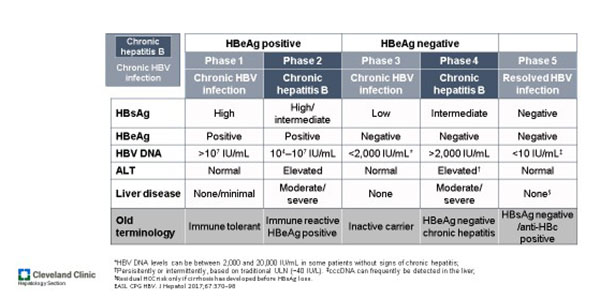
It is important to point out that Phase 5 (“resolved HBV infection”) should not be interpreted as absence of hepatitis B virus fragments. Once infected, most individuals harbor HBVDNA within the hepatocyte cell nucleus forever, either integrated into host DNA or as cccDNA, a free standing “mini-chromosome.” Under circumstance described later, reactivation of hepatitis B (reversion to Phase 2 or 4) may occur with severe consequences.
Those with immune-reactive HBeAg positive chronic hepatitis B (Phase 2) and HBeAg-negative chronic hepatitis B (Phase 4) are most likely to have active liver injury and therefore be candidates for antiviral treatment.
Spontaneous loss of HBeAg occurs at a rate of 8% to 12% per year, and is associated with a decrease in HBV DNA. Loss of HBsAg occurs less often (<1%/year). Chronically infected individuals without active liver disease or viral replication (inactive carriers) generally have a benign course, with a smaller likelihood of progressing to cirrhosis. Those who continue to have active viral replication with high levels of HBV DNA and HBeAg in serum often have progressive liver injury, and cirrhosis and end-stage liver disease can develop. A transient flare of disease often precedes remission.
Loss of HBeAg is not always followed by permanent resolution of disease and disease flares can occur, particularly if a patient is treated with steroids or other immunosuppressive medications. Patients who revert to chronic HBeAg-positive status tend to develop cirrhosis at a substantially increased rate compared with those who remain HBeAg-negative. HBe Ag-negative individuals with evidence of ongoing hepatic inflammation, tend to have a higher risk of disease progression than patients who are HBeAg-positive.
Hepatocellular carcinoma: Chronic HBV infection is associated with a ten-fold increase in the risk of developing hepatocellular carcinoma (HCC). This risk is further magnified in the setting of ongoing inflammation: In those with both HBsAg and HBeAg, the risk increases to 60-fold compared with the general population. Older men with cirrhosis and those co-infected with hepatitis C are at greatest risk. In regions where HBV is endemic, HCC is the leading cause of cancer-related death.
Twice yearly ultrasound (with or without alpha fetoprotein) screening for HCC is recommended for the following individuals with chronic hepatitis B:
- Cirrhosis
- Asian or African-born black men over age 40
- Asian women over age 50
- First degree relative of those with HCC
- Coinfection with HIV or hepatitis D
Absent access to ultrasound alpha fetoprotein alone may be used. HCC.
Treatment
In typical acute hepatitis B, treatment is supportive. There is no clear evidence that early therapy with antiviral agents for acute hepatitis B decreases the risk of chronicity or speeds recovery. Most with acute icteric hepatitis B recover without residual injury or chronic hepatitis. These individuals should be followed with repeat testing for HBsAg and ALT levels to determine whether seroconversion and clearance of surface antigen have occurred. An exception is made in the individual with fulminant hepatitis B in who a liver transplant is contemplated. In such cases antiviral therapy is usually initiated.
The following situations call for initiation of hepatitis B antiviral therapy:
- Fulminant hepatitis B in whom liver transplantation is contemplated
- Immune reactive HBeAg positive chronic hepatitis B (Phase 2)
- HBeAg-negative chronic hepatitis (phase 4)
- Pregnant women whose viral load exceeds 200,000 IU
- Those at risk for reactivation hepatitis B.
Currently available antiviral drugs do not cure hepatitis B, or even speed loss of HBsAg. Therapy is able to suppress viral replication and liver inflammation, and to prevent or reverse liver fibrosis. Therapy may also induce seroconversion from Phase 2 (HBeAg positive immune reactive chronic hepatitis) to a more quiescent Phase 3 (HBe Ag negative inactive carrier).
Most often, a decision to treat chronic hepatitis is made without a liver biopsy, even though a biopsy provides the most accurate information about necroinflammatory activity and fibrosis.
In those with cirrhosis antiviral treatment is recommended when there is measurable HBV DNA regardless of liver enzymes, and HBeAg status. This approach is supported by several studies that have shown a decreased rate of development of progressive liver disease or complications in treated patients.
Pregnant women are screened for hepatitis B, so that newborns of HBsAg positive mothers can be given active immunization and HBIG, thereby reduce the likelihood of vertical transmission. It has recently been shown that added protection is afforded newborns if mothers with a very high viral load (> 200,000 IU) are treated with and NA during the third trimester to reduce viral load.
Reactivation Hepatitis B occurs with surprising frequency in susceptible individuals undergoing therapy for cancer or rheumatologic diseases. All individuals in whom treatment with cytotoxic, immunosuppressive or immunomodulatory therapy should be tested for HBsAg, and anti HBc. Preemptive nucleoside treatment should be provided for.
|
Pre-emptive Hepatitis B Prophylaxis in Those At Risk For Reactivation |
|
|
HBsAg +/ anti HBc + |
HBsAg-/Anti HBc + |
|
Immunosuppressive therapy |
Anti CD-20 therapy (e.g, rituximab) |
|
Cytotoxic therapy |
Stem cell transplantation |
The AASLD guideline allows for careful monitoring (frequent ALT, HBV DNA, HBsAg) those with only anti HBc not receiving anti CD-20 therapy or stem cell transplantation. Because reactivation hepatitis B may be associated with severe disease, liver decompensation, and high likelihood of death, we advise management of HBsAg-/anti HBc positive in a fashion identical to those who are HBsAg positive.
Pharmacologic Treatment
Six antiviral agents have been approved by the U.S. Food and Drug Administration (FDA) to treat hepatitis B. Only 3 are commonly used in the United States, all nucleoside or nucleotide analogues (NA)
- Tenofovir alafenamide 25 mg per day (adults)
- Tenofovir disosoproxil 300 mg per day (adults)
- Entecavir 0.5 to 1 mg per day (adults)
Each is excreted by the kidney so dose reductions are required for those with impaired glomerular filtration rates. Each reliably causes remarkable reduction in viral loads and viral resistance is rare. Optimal duration of treatment continues to be a matter of debate for those with Phase 2 hepatitis B who have seroconverted from HBeAg positive to anti HBe positive. Some may remain inactive carriers if NA treatment is stopped 6-12 months after HBe seroconversion. Because many flare, treating providers are often loathe to stop NA. There is more consensus about the need for permanent NA therapy in those with Phase 4 hepatitis B. An exception to life-long treatment is made in those fortunate few who become HBsAg negative and remain so for a period of six months.
Other NAs are plagued by emergence of viral resistance and are rarely used.
Interferon alfa therapy for hepatitis B has been FDA-approved since 2005. Because it requires subcutaneous injections and is associated with a myriad of side effects. The major side effects of interferon include fatigue, muscle aches, fever, depression, and irritability. Uncommon severe side effects include exacerbation of depression, psychosis, renal and cardiac failure, bacterial infections, and induction of autoimmunity. Patients with a beneficial response to interferon therapy often develop a flare of disease, with elevations of serum ALT to levels two to three times the baseline before normalization occurs. Because of the possibility that a flare of liver disease can lead to decompensation, the use of interferon in cirrhotic patients is not recommended. Long courses of interferon treatment are unrivaled in achieving loss of HBsAg. Nevertheless, this treatment is rarely used in the US today.
Although the introduction of nucleotide or nucleoside analogues represents a significant advance in the management of chronic hepatitis B, many questions remain regarding optimal dosing, duration, and possible combinations to prevent resistance, increase long-term suppression. These emerging therapies, including newer and more potent antiviral agents, coupled with aggressive worldwide vaccination policies, lend promise to the hope that hepatitis B will one day be controlled. Indeed, in 2015 the World Health Organization in developed a realistic plan to eliminate hepatitis B throughout the world by 2030.
Other Treatment considerations
Considerable evidence has accumulated that those with chronic hepatitis B who receive lipophilic statins (atorvastatin, simvastatin, lovastatin, fluvastatin, cerivastatin and pitavastatin) are at a remarkably lower risk for development of hepatocellular carcinoma. Such agents should be considered even in the absence of elevated lipids.
Summary
- Hepatitis B is found throughout the world. Its incidence is especially high in Asia, sub-Saharan Africa, the South Pacific, South America, the Middle East, and the Arctic.
- The most common mode of transmission of hepatitis B worldwide is from mother to infant. Hepatitis B is spread predominantly parenterally, through intimate personal contact, and perinatally.
- Persons at risk include intravenous drug users, children of mothers with HBV, men who have sex with men, patients on hemodialysis, and those exposed to contaminated blood or blood products.
- Most acute infections produce no symptoms. When present, symptoms range widely in severity, from asymptomatic subclinical infection to fulminant fatal disease.
- The risk of developing chronic infection (or the carrier state) depends on the age and immune function of the patient at the time of initial infection.
- Viral and immune markers are detectable in blood, and characteristic antigen-antibody patterns evolve over time. The first detectable viral marker is HBsAg, followed by HBeAg and HBV DNA.
- Effective vaccines for HBV, defined as inducing better than 90% protection against HBV, have been available in the United States since 1982. Hepatitis B vaccine has been described as the first effective anticancer vaccine, and its use has been promoted by the World Health Organization for routine care worldwide since 1997.
- In acute hepatitis B, treatment is supportive. Although several case series have been published, there is no clear evidence that early therapy with antiviral agents for acute hepatitis B decreases the risk of chronicity or speeds recovery. In chronic hepatitis B, therapy is administered to suppress viral replication and prevent progression of liver disease. Many treatment programs have been shown to be effective and have been approved by the FDA and other governmental health agencies around the world
Acknowledgement: This chapter builds on a previous version written by Robert O’Shea MD
Suggested Reading
- Terrault N, Lok A, McMahon J, et al. Update on the prevention, diagnosis and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. HEPATOLOGY, VOL. 67, NO. 4, 2018; 1560 (https://www.aasld.org/sites/default/files/2019-06/HBVGuidance_Terrault_et_al-2018-Hepatology.pdf)
- Cox, A.L., El-Sayed, M.H., Kao, JH. et al. Progress towards elimination goals for viral hepatitis. Nat Rev Gastroenterol Hepatol 17, 533–542 (2020). https://doi.org/10.1038/s41575-020-0332-6
- Hepatitis B Questions and Answers for Health Professionals (https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm)