BioRepository Resources & Services
Mission
To safely maximize the value of human samples by supplying high quality, well-annotated specimens to researchers whose discoveries will be essential for the evolution of personalized medicine.
Resources
- Blood and blood products (DNA, RNA, packed cells, plasma, serum, PBMCs).
- Urine.
- Stool.
- Tissue (including paraffin blocks, frozen sections and fresh frozen).
- Key medical record data.
- Patient-reported outcomes.
Services
The following services are available for projects that have been approved by Cleveland Clinic’s BioRepository Review Committee.
- Identification and collection of residual tissue and blood or prospectively collected blood and other bodily fluids (urine, saliva, joint fluid, ascites, etc.) using uniform operating procedures and standardized collection kits.
- Pre-analytic specimen processing under a standardized and annotated process in a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory.
- Phenotypic data collection for each sample procured.
- Quality assurance of specimens according to College of American Pathology (CAP) guidelines.
External Collaborations
Please note that external researchers must have a collaborator from Cleveland Clinic to request the resources and services outlined above. All services/collaborations will need to be approved by CCBioR Review Committee. For more information, please contact Cleveland Clinic’s BioRepository at 216.445.1123.
For Cleveland Clinic researchers who wish to use the CC-BioR, visit the BioRepository intranet.
Enrollment & Demographics
Specimens are collected via enrollment in three parallel paths:
- Surgery
The collection of paired tissue and blood. These are redundant specimens, meaning all clinical testing has been resulted and the material would otherwise be discarded. - Outpatient Labs
The collection of residual blood. These are redundant specimens, meaning all clinical testing has been resulted and the material would otherwise be discarded. - Active Recruitment*
The collection of fresh samples. These are fresh specimens collected for research only, facilitating proteomics, RNA and cell-based studies that require fresh samples.
*In May 2020 we began collecting blood, urine and stool from COVID-19 positive patients and have since expanded our collection to include a COVID-19 negative cohort as well. These patients are included in the demographics summarized below.
Demographics
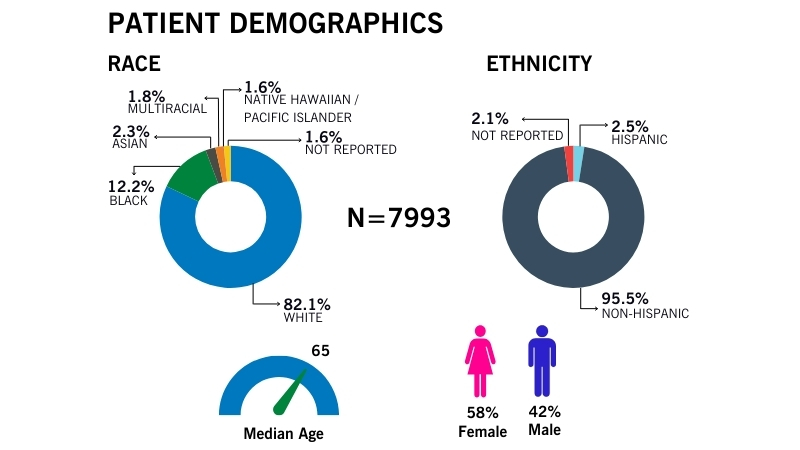
Cleveland Clinic’s BioRepository aims to collect specimens from a diverse pool of participants to reflect the diversity in our communities.
We collect specimens that are varied in age, sex, race and clinical diagnoses.
View recent demographics (updated June, 2024). To access more recent data, please contact us.