What is PML and what causes it?
At the Mellen Center, we can see PML in multiple sclerosis (MS) patients who are JC virus (JCV) positive and on disease modifying therapies known to increase the risk of PML, specifically natalizumab. Progressive multifocal leukoencephalopathy (PML) is a rare but serious brain infection that is caused by the JC virus (JCV). It is estimated that at least 50% of the general population has been exposed to JCV, but infection is generally asymptomatic in immunocompetent individuals.1, 2 However, in immunocompromised patients, including those taking certain multiple sclerosis disease modifying therapies (DMT), JCV can mutate and infect the brain, specifically oligodendroglial cells, and result in PML. Other possible central nervous system manifestations of JCV include cerebellar granule cell neuronopathy, encephalopathy, and meningitis.3, 4
When should a clinician caring for an MS patient suspect PML?
PML should be suspected in MS patients with new characteristic lesions on MRI or sub-acute (evolving over weeks or months) progressive neurologic symptoms in the setting of immunosuppressive medications known to increase the risk of PML. PML should be considered in patients who develop atypical MS lesions or otherwise typical new lesions for MS while on natalizumab. The clinical symptoms of PML can vary significantly between patients, and may include weakness, paresthesias, cognitive or behavioral changes, gait dysfunction, speech/language difficulties, visual field defects, or seizure. However, PML can be asymptomatic for many months prior to clinical presentation with new lesions on MRI, so patients should be monitored closely via MRI and undergo lumbar puncture for JCV PCR if there is a suspicion for PML.
Anti-JCV Antibody Positive
| Anti-JVC Antibody Negative |
Natalizumab exposure (months) |
Patients w/o prior immunosuppressant use |
| <1/1000 | 1-24 | <1/1000 |
| 25-48 | 3/1000 | |
| 49-72 | 6/1000 |
| Anti-JVC Antibody Negative |
Natalizumab exposure (months) |
Patients with prior immunosuppressant use |
| <1/1000 | 1-24 | <1/1000 |
| 25-48 | 12/1000 | |
| 49-72 | 13/1000 |
If PML is suspected, clinicians should hold DMT pending further evaluation, depending on the level of suspicion. A clinician may repeat a brain MRI in 1 month if a new subcortical lesion develops, but further immediate evaluation with lumbar puncture for JCV PCR is reasonable depending upon the patient’s JCV antibody status and clinical history. Although serum JCV antibody testing can help assess the risk of PML, it does not indicate the presence or absence of PML.
How is PML diagnosed?
The diagnosis of PML depends on integration of imaging, clinical, and laboratory evidence, as there is not a single test that establishes a diagnosis of PML (Table 1).2
Brain MRI most commonly demonstrates one or more T2/FLAIR hyperintense and T1 hypointense lesions involving the subcortical and juxtacortical white matter (Figure 1). Up to half of patients with MS disease modifying therapy-associated PML exhibit faint rim enhancement with gadolinium administration, so this is not uncommon at the time of PML diagnosis.2, 6 PML lesions can be multifocal or unifocal. The dynamic nature of MRI findings can contribute to diagnosis, since PML becomes unlikely if the MRI manifestations are stable over weeks to a few months on serial imaging. Both PML and an inflammatory response to the virus will result in dynamic changes on MR images, while static findings are inconsistent with this disease.
Figure 1 – Typical MRI findings of PML
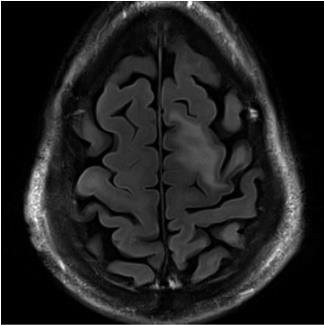
CSF examination is an integral component of evaluation for PML, but CSF JCV PCR testing methods have variable sensitivity. The Cleveland Clinic Laboratory JCV PCR is a send out test to the Mayo Clinic, which reports a lower limit of detection of 10 copies/microliter of CSF.7 Another ultra-sensitive multiplex JCV PCR is available via the National Institutes of Health.8 If PML is highly suspected and the Mayo Clinic CSF JCV PCR is negative, one should repeat the lumbar puncture for ultra-sensitive PCR at the NIH, which requires frozen samples. The Cleveland Clinic lab typically does not freeze CSF upon receipt, so specific instructions are necessary (see Appendix).
Although diagnosis can generally be established via clinical history, MRI, and CSF analysis, brain biopsy is sometimes obtained when the diagnosis of PML has not been confirmed. Prior to biopsy it is worthwhile to repeat the CSF sampling at least once unless the rate of progression suggests the need to immediately sample tissue in an effort to find an alternative diagnosis. Histopathological examination of brain tissue confirming PML will demonstrate characteristic demyelination, bizarre astrocytes, and oligodendroglial nuclear inclusions seen with PML. These findings should be confirmed with specific histopathology or molecular analysis. Tissue PCR for JCV is also performed in biopsy specimens to support association of the brain lesion with JC virus.
Table 1 – Evidence supporting the diagnosis of PML
| Modality | Finding(s) |
|---|---|
| Clinical history | Subacute onset of weakness, paresthesias, cognitive or behavioral changes, gait dysfunction, speech/language difficulties, or seizure |
| Brain MRI | ≥1 T2/FLAIR hyperintense and T1 hypointense lesions involving the subcortical and juxtacortical white matter, some may exhibit faint rim enhancement Atypical MS lesions or even typical lesions for MS while on natalizumab |
| Cerebrospinal fluid | CSF JCV PCR (commercial or ultrasensitive/quantitative PCR at NIH) positive |
| Brain biopsy | Histopathology: demyelination, bizarre astrocytes, and oligodendroglial nuclear inclusions Tissue PCR for JCV positive |
What are mimics of PML that should be considered in this evaluation?
In the process of evaluating a patient with suspected PML, other etiologies to consider include Posterior Reversible Encephalopathy Syndrome (PRES), CNS vasculitis, VZV leukoencephalitis, neoplasm (metastases, glioma, and lymphoma), HSV encephalitis (especially in the setting of seizure and temporal lobe lesions), autoimmune encephalitis, and multiple sclerosis relapse.2
What is the appropriate treatment for natalizumab-associated PML?
Natalizumab should be immediately discontinued, and the patient should be hospitalized for urgent plasmapheresis. Plasmapheresis expedites clearance of natalizumab, accelerating restoration of leukocyte transmigration across the blood brain barrier.9 A course of five 1.5-volume exchanges, performed every other day, is recommended to ensure that natalizumab levels are reduced sufficiently to allow lymphocyte migration into the CNS. Modern apheresis management typically uses lower volume exchanges (i.e. 1-volume), which may then require more exchanges. These protocol differences should be discussed with laboratory medicine. Other CNS manifestations of JCV infection (cerebellar granule cell neuronopathy, meningitis, and encephalitis) should be treated similarly.3, 4 However, the impact of plasmapheresis on long-term outcomes is somewhat unclear.10-12
What is the appropriate treatment for PML in the setting of other MS DMTs?
Rare cases of PML have been reported with use of other MS DMTs, both independent of and following use of natalizumab. In the setting of monoclonal-antibody associated PML (including rituximab or ocrelizumab), plasmapheresis should be considered if PML is diagnosed within 2 months of drug administration, but the evidence supporting accelerated drug clearance is not as strong as with natalizumab. For PML associated with other MS DMTs (including fingolimod and dimethyl fumarate), immediate discontinuation of the immunosuppressive agent is recommended. If PML is diagnosed in the setting of teriflunomide, an accelerated drug clearance protocol (for example, activated charcoal administration) should be instituted. There is no current evidence supporting the use of IVIg or GM-CSF in treatment of PML. However, in anti-CD20-associated cases of PML, one could consider IVIg administration if a patient has low levels of IgG. This management strategy also applies to other CNS manifestations of JCV infection.
What is the evidence supporting use of other medications (mirtazapine, maraviroc, and mefloquine) in management of PML?
Several medications have demonstrated in vitro effects against JCV replication and cell entry, including mirtazapine and mefloquine. However, the evidence for their clinical effectiveness is limited.13, 14 Maraviroc, a CCR5 chemokine receptor antagonist, is hypothesized to reduce PML-associated IRIS, and is discussed further below.15 Of these medications, mirtazapine appears to be the most promising, particularly in natalizumab-associated PML.16 Given its potential survival benefit and low likelihood of side effects other than somnolence, we recommend initiation of mirtazapine 30 to 45mg daily, with a maximum dosage of 60mg daily (Table 2). We do not recommend routine use of mefloquine at this time due to potential psychotropic side-effects.
Table 2 – Recommended Dosing of Adjuvant Medications in PML Management
| Drug | Proposed Mechanism | Dose |
|---|---|---|
| Mirtazapine | Possibly inhibits JCV replication and cell entry | 30 to 45mg daily, with a maximum dosage of 60mg daily |
| Maraviroc | CCR5 antagonist, potentially reducing severity of IRIS* | 300mg twice a day |
* Immune Reconstitution Inflammatory Syndrome
What is IRIS and why should we be concerned about it?
Although immune reconstitution is desired for management of PML and results in better outcomes, Immune Reconstitution Inflammatory Syndrome (IRIS) is a potentially dangerous amount of acute and sometimes fulminant inflammation in the brain that should be treated.14 IRIS usually follows withdrawal of immunosuppression and causes clinical deterioration associated with the patient’s immune response reacting to the newly-recognized JCV infection in the CNS. Severe IRIS is generally characterized by both clinical and radiographic worsening.
Mellen Center Approach: PML Diagnosis & Management
Brain MRI may demonstrate gadolinium enhancement at the site of PML lesions due to breakdown of the blood brain barrier from the inflammatory response. The increasing degree of inflammatory activity seen with IRIS portends poor prognosis, and therefore warrants treatment.11, 14 Specifically, the immune response to JCV-infected oligodendroglia is hypothesized to injure uninfected oligodendroglia. It is also important to consider early MS disease activity as a possibility in the months following PML, but within 6 months of diagnosis MRI changes are likely related to PML and/or IRIS.
What is the treatment for PML-related IRIS?
Generally, IRIS is managed with intravenous methylprednisolone to suppress the immune response and therefore its potentially dangerous brain inflammation. Given the need for immune reconstitution for control of JCV infection, the balance between immune response against JCV infection and immunosuppression to moderate IRIS can be challenging.14 If a patient with PML develops clinical symptoms of IRIS and MRI confirms inflammation (i.e. increase in T2 hyperintensities, usually with gadolinium enhancement), IV methylprednisolone should be administered (1g IV daily for 3 days). Periodic clinical and MRI reassessment should be performed to follow response to therapy, and IV methylprednisolone can be repeated every 4 weeks if needed for clinical manifestations of IRIS. Isolated MRI changes without clinical worsening do not necessarily warrant change in management.
Maraviroc, a CCR5 chemokine receptor antagonist, is hypothesized to reduce the severity of IRIS by decreasing recruitment of CCR5 positive lymphocytes into the central nervous system.14, 15 The clinical effectiveness of maraviroc has not been established, though there are case reports suggesting potential benefit.17, 18 Potential side effects include hepatotoxicity, infections, and skin hypersensitivity reactions, which can be severe. We recommend starting maraviroc, 300mg twice a day, at the time of PML diagnosis.
What are the common complications of PML?
Depression is commonly seen in patients who develop PML and should be treated with antidepressants and psychotherapy. Given the potential antiviral benefit of mirtazapine, this is often considered as a first-line treatment of depression. Seizures are occasionally seen, although can be subtle, particularly if they arise from the frontal or temporal lobes. Although some clinicians utilize antiepileptic mediations prophylactically, we generally advise against this approach and instead start antiepileptic medications only after a confirmed seizure has occurred.
What is the prognosis of PML secondary to MS therapies?
If patients can overcome the initial PML infection and subsequent IRIS, then the long-term prognosis is variable but can be good. In general, residual neurological deficits are common. In patients with natalizumab-associated PML, survival is estimated to be approximately 75% overall.19, 20 Factors associated with better prognosis include younger age at diagnosis and lower pre-PML disability. Early recognition and diagnosis of asymptomatic PML with appropriate management also portend better prognosis.19 However, clinical stability typically takes 3-6 months after the diagnosis of PML is made.20
Appendix: Lab procedures for NIH JCV PCR testing
If a clinician desires to send a CSF sample for ultrasensitive JCV PCR at the NIH, several additional steps must be taken:
- The Cleveland Clinic lab should be instructed to freeze at least a 1ml aliquot of CSF upon receipt, and that it must be frozen for transport
- The clinician should place an order for an extra tube, and the information in the comments section should be populated by the Epic SmartPhrase “.nihjcv,” which should be available to all Mellen Center physicians. This SmartPhase contains all necessary information about the test, sample handling, and shipping instructions.
- A specific form for the NIH must be completed regarding the patient’s history and sample being sent, and should be sent to the Cleveland Clinic lab with the sample. This forms is available on the Cleveland Clinic Neurological Institute shared drive at “S:\NEURO\PUBLIC\Mellen JCV testing at NIH forms”, along with detailed shipping instructions for the sample.
Acknowledgements:
We would like to thank Dr. David Clifford of Washington University in St. Louis for his involvement in development of this Mellen Approach.
References:
- Bozic C, Subramanyam M, Richman S, et al. Anti-JC virus (JCV) antibody prevalence in the JCV Epidemiology in MS (JEMS) trial. Eur J Neurol 2014; 21: 299-304. 2014/06/05. DOI: 10.1111/ene.12304.
- Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology 2013; 80: 1430-1438. 2013/04/10. DOI: 10.1212/WNL.0b013e31828c2fa1.
- Tan CS and Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. The Lancet Neurology 2010; 9: 425-437. 2010/03/20. DOI: 10.1016/s1474-4422(10)70040-5.
- Schippling S, Kempf C, Buchele F, et al. JC virus granule cell neuronopathy and GCN-IRIS under natalizumab treatment. Annals of neurology 2013; 74: 622-626. 2013/07/23. DOI: 10.1002/ana.23973.
- H K, I C, B S, et al. New Algorithm to Estimate Risk of Natalizumab-Associated Progressive Multifocal Leukoencephalopathy (PML) in Anti-JCV Antibody Positive Patients: Analyses of Clinical Trial Data to Provide Further Temporal Precision and Inform Clinical Practice 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) 2016.
- Clifford DB, De Luca A, Simpson DM, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. The Lancet Neurology 2010; 9: 438-446. 2010/03/20. DOI: 10.1016/s1474-4422(10)70028-4.
- Mayo Medical Laboratories. LCJC - Clinical: JC Virus, Molecular Detection, PCR, Spinal Fluid, https://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/88909 (2017).
- Ryschkewitsch CF, Jensen PN and Major EO. Multiplex qPCR assay for ultra sensitive detection of JCV DNA with simultaneous identification of genotypes that discriminates non-virulent from virulent variants. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 2013; 57: 243-248. 2013/04/27. DOI: 10.1016/j.jcv.2013.03.009.
- Khatri BO, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology 2009; 72: 402-409. 2009/02/04. DOI: 10.1212/01.wnl.0000341766.59028.9d.
- Landi D, De Rossi N, Zagaglia S, et al. No evidence of beneficial effects of plasmapheresis in natalizumab-associated PML. Neurology 2017; 88: 1144-1152. 2017/02/24. DOI: 10.1212/wnl.0000000000003740.
- Tan IL, McArthur JC, Clifford DB, et al. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology 2011; 77: 1061-1067. 2011/08/13. DOI: 10.1212/WNL.0b013e31822e55e7.
- Tyler KL and Vollmer TL. To PLEX or not to PLEX in natalizumab-associated PML. Neurology 2017; 88: 1108-1109. DOI: 10.1212/wnl.0000000000003747.
- Clifford DB, Nath A, Cinque P, et al. A study of mefloquine treatment for progressive multifocal leukoencephalopathy: results and exploration of predictors of PML outcomes. J Neurovirol 2013; 19: 351-358. 2013/06/05. DOI: 10.1007/s13365-013-0173-y.
- Clifford DB. Progressive multifocal leukoencephalopathy therapy. J Neurovirol 2015; 21: 632-636. 2014/09/18. DOI: 10.1007/s13365-014-0289-8.
- Giacomini PS, Rozenberg A, Metz I, et al. Maraviroc and JC virus-associated immune reconstitution inflammatory syndrome. The New England journal of medicine 2014; 370: 486-488. 2014/01/31. DOI: 10.1056/NEJMc1304828.
- Jamilloux Y, Kerever S, Ferry T, et al. Treatment of Progressive Multifocal Leukoencephalopathy With Mirtazapine. Clinical drug investigation 2016; 36: 783-789. 2016/07/13. DOI: 10.1007/s40261-016-0433-8.
- Martin-Blondel G, Cuzin L, Delobel P, et al. Is maraviroc beneficial in paradoxical progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome management? Aids. England, 2009, pp.2545-2546.
- Scarpazza C, Prosperini L, Mancinelli CR, et al. Is maraviroc useful in multiple sclerosis patients with natalizumab-related progressive multifocal leukoencephalopathy? J Neurol Sci 2017; 378: 233-237. 2017/06/02. DOI: 10.1016/j.jns.2017.05.018.
- Dong-Si T, Richman S, Wattjes MP, et al. Outcome and survival of asymptomatic PML in natalizumab-treated MS patients. Annals of clinical and translational neurology 2014; 1: 755-764. DOI: 10.1002/acn3.114.
- Dong-Si T, Gheuens S, Gangadharan A, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol 2015; 21: 637-644. 2015/03/17. DOI: 10.1007/s13365-015-0316-4.